Objectives
Mantle cell lymphoma (MCL) has poor prognosis with existing available therapies. Zanubrutinib, a novel BTK inhibitor, has shown promising outcomes in relapsed/refractory MCL. The aim of this retrospective study was to evaluate the efficacy and safety of zanubrutinib combined with R2-CHOP (lenalidomide, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in newly diagnosed MCL.
Methods
We retrospectively analyzed newly diagnosed MCL between September 2020, to March 2023 in a single-center in China. All patients received zanubrutinib 160 mg twice daily, lenalidomide 25 mg daily (days 1 to day 14), rituximab 375 mg/m 2, cyclophosphamide 750 mg/m 2 (day 1), doxorubicin 50 mg/m 2 or liposome doxorubicin 40 mg (day 1), vincristine 1.4 mg/m 2 (day 1), and prednisone 100 mg (day 1 to day 5) every 3 weeks. After 4 cycles, autologous hematopoietic stem cell transplantation (ASCT) was performed according to the patient's condition. After 6 cycles of induction or ASCT, patients with an objective response (complete or partial response) continued to receive zanubrutinib twice daily plus rituximab maintenance therapy, administered every 12 weeks for up to 8 additional doses. All patients underwent CT or PET-CT scans to evaluate mid-term efficacy. After 6 cycles, PET-CT scans were evaluated for patients whose mid-term efficacy did not achieve complete metabolic response (CMR).
Results
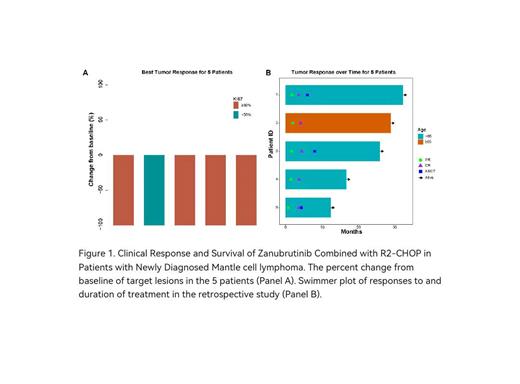
All of 5 patients treated with ZR2-CHOP were enrolled. The median age was 50 years (range: 44-66 years), 3 patients were females, 2 patients were males. 3 patients were classical MCL, 1 patient was pleomorphic MCL, and 1 patient was blastoid-variant MCL. The overall response rate was 100% (5 of 5), and all of them achieved CMR (Figure 1). Three patients achieved CMR after 4 cycles received ASCT, two received 6 cycles. At the mid-term evaluation after 4 cycles, 4 patients achieved CMR, 1 patient achieved PR but achieved CMR after 6 cycles. At data cutoff (22 March 2023), the median follow-up time was 26.1 (range, 12.5-32.4) months. The median PFS and OS rates were not reached. The 2-year PFS rate and the 2-year OS rates were both 100% (Figure 2). The most common grade 3/4 adverse events were neutropenia (60%) and thrombocytopenia (40%), all of which were controllable and resolved. The results of WES analysis showed that the most common gene mutations were KMT2D (frequency, 60%) and DDR2 (frequency, 40%). ctDNA was dynamically detected in four patients. Matched analysis of WES and ctDNA showed that gene mutations detected by these two methods were highly consistent, with 3 out of 4 patients being consistent. Among them, one showed negative ctDNA before and after treatment, while the other three patients indicated a decreasing trend in ctDNA mutation rate after 4 or 6 cycles, including a 50% decline or a change from baseline to negative.
Conclusions:
ZR2-CHOP showed promising preliminary efficacy and acceptable safety in previously untreated patients with MCL.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Zanubrutinib, a novel BTK inhibitor, has shown promising outcomes in relapsed/refractory MCL. The aim of this retrospective study was to evaluate the efficacy and safety of zanubrutinib combined with R2-CHOP (lenalidomide, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in newly diagnosed MCL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal