Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous and invasive lymphoma. About 30% of patients are difficult to cure or relapse after R-CHOP treatment, known as relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL). Patients who are not suitable for high-dose chemotherapy followed by autologous stem cell rescue, rely more on the discovery of new drugs and proposals of new treatment plans. Polatuzumab vedotin (Pola) is an antibody-drug conjugate that targets the B-cell antigen CD79b and delivers monomethyl auristatin E (MMAE). It is recently available in China and approved in combination with Bendamustine and Rituximab for r/r DLBCL. However, real-world data on Pola-based regimens are limited. Here, we propose ZPR (combination of Zanubrutinib, Polatuzumab vedotin and Rituximab) as a potential salvage treatment in r/r DLBCL patients, aiming to explore its efficacy and safety in our single center.
Response characteristics in patients received ZPR therapy
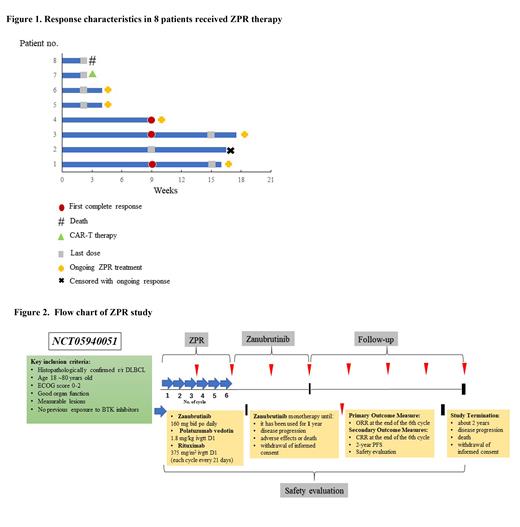
There were already eight r/r DLBCL patients treated with ZPR regimen at our institution between April 1 and July 31, 2023. Informed consent and biological sample donation informed consent were both signed by each participant. Patients were 69 years old on median and half of them were non-GCB type. Seven patients are classified as stage IV. The median number of treatment lines is two. Patients were given Zanubrutinib 160mg bid daily; Polatuzumab vedotin 1.8 mg/kg and Rituximab 375 mg/m 2 on the first day of each cycle (every 21 days per cycle). Four patients completed 3 cycles of ZPR and three underwent a mid-term evaluation. Results showed that the complete remission (CR) rate was 100% (Figure 1). In terms of adverse events, hematological toxicity grade is not greater than level 2 of Common Terminology Criteria for Adverse Events (CTCAE). One patient developed a pulmonary infection during the treatment.
The Rationale for the combination of Polatuzumab vedotin, Rituximab and Zanubrutinib
The combination therapy of bruton's tyrosine kinase inhibitor (BTKi) with anti-CD20 therapy has been used in many B-cell lymphomas. It was proposed that combining these two drugs will target and clear blood lymphocytes and shorten the time to respond in chronic lymphocytic leukemia patients. Compared with the first generation BTKi the new generation BTKi Zanubrutinib has a higher selectivity and fewer off-target effects on BTK. Meanwhile, Natsumi Kawasaki et al discovered that Pola increased the expression of CD20 and sensitized Pola refractory cells to Rituximab-induced complement-dependent cytotoxicity (CDC). While the release of MMAE, a chemical component of Pola, results in microtubule disruption and cycle arrest. These findings provide a scientific rationale for employing the Pola-Rituximab combination therapy to treat DLBCL patients. However, the combination of Pola and Zanubrutinib does not show a clear mutual antagonism effect. The molecular rationale for the combination of these two drugs needs further exploration.
Who are suitable for ZPR regimen?
Patients who are diagnosed with r/r DLBCL after standard first-line treatment failure:
ECOG≥2
Age≥70
As a bridging therapy for patients waiting for ASCT or CAR-T therapy
Age<70 expect a high quality of life, still need working, or going to school
Unable to tolerate standard-dose of chemotherapy due to hematological toxicity (need a blood transfusion, treatment delay, or dosage reduction)
Prospective study to be continued
Due to the good response of our three r/r DLBCL patients, we intended to conduct a “Prospective single-arm, single-center clinical study of Zanubrutinib, Polatuzumab vedotin and Rituximab (ZPR) regimen in relapsed/refractory patients with diffuse large B-cell lymphoma” to learn more about the impact of the ZPR regimen in the treatment of r/r DLBCL. We got the approval from the Investigator-Initiated Trial (IIT) Scientific Review Board of Zhongshan Hospital after discussing the risks and benefits of ZPR regimen with them. This protocol has also been reviewed and approved by the Institutional Review Board (IRB) of Zhongshan Hospital Affiliated to Fudan University (B2023-186R) and will follow the principles of the Helsinki Declaration. The protocol was previously registered on ClinicalTrials. gov (NCT05940051) . We illustrated the flowchart of our study in Figure 2.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Zanubrutinib in treatment of DLBCL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal