Introduction: Treatment-free remission (TFR) as one of the goals of treatment of BCR::ABL-positive chronic phase chronic myeloid leukemia (CML) has been proposed. Actually, approximately 40-60% of CML patients maintain TFR without recurrence after discontinuation of tyrosine kinase inhibitors (TKIs). In some of such patients, there are fluctuated cases in which BCR::ABL positive clones continue to be detected for long periods at levels of international scale (IS) BCR::ABL < 0.1%. Such fluctuated cases show no apparent molecular recurrence and maintain TFR for a long time, but they have also been shown to be more likely to eventually develop late recurrence (Haematologica 2022, Blood adv. 2020). We have reported that a higher percentage of PD-1 positive CD8 + T cells before and after TKI discontinuation is associated with molecular recurrence, and together with the possibility of assessing host immune response capacity after TKI discontinuation by using regulatory T (Treg) cells (Fujioka, et al. Cancers 2021). On the other hands, mutations in BCR::ABL caused by the alternative splicing, such as BCR::ABL Ins35bp, are known to be one of the TKI resistance in CML. Loss of BCR::ABL kinase activity is thought to result in loss of TKI sensitivity and thus failure to achieve deep molecular remission (Yuda, et al. Cancer Science 2017). In this study, we analyzed the mutations that cause splicing variants such as BCR::ABL Ins35bp and host immune response in CML patients to elucidate molecular mechanisms in the long-term maintenance of TFR and late recurrence.
Methods: Twenty-four CML patients who discontinued TKI treatment for any reason at Akita University Hospital were included in the study. The trends of IS level after TKI discontinuation were monitored, and immune profile analysis by multi-color flow cytometry (FCM) was performed in all patients. Mutation analysis of the BCR::ABL region including splicing variants was performed in selected patients. Mutation analysis was performed on samples with sufficient cell count and IS > 0.0032% (MR 4.5), and the BCR::ABL region of these samples was amplified by long nested PCR and then analyzed by the next-generation sequencer (NGS).
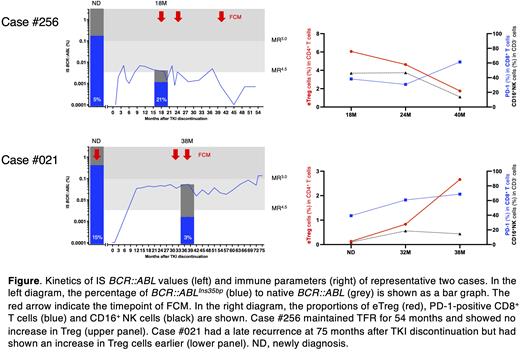
Results: First, BCR::ABL Ins35bp was evaluated by mutation analysis. Among CML patients who underwent mutation analysis, BCR::ABL Ins35bp was detected in all cases in which the samples could perform analysis at newly diagnosis (ND). Even after TKI discontinuation, a certain percentage of BCR::ABL Ins35bp was detected in all patients, and the percentage of BCR::ABL Ins35bp increased at the time of recurrence regardless of whether early or late. These results suggest that function-dead BCR::ABL Ins35bp clones may remain in CML patients with detectable BCR::ABL under and after TKI treatment, and eventually leading to recurrence with transcriptional promotion of native BCR::ABL. On the other hand, there were cases in which the BCR::ABL Ins35bp was lost at the time of recurrence. There is a possibility of immunological involvement in such cases. CML patients with detectable BCR::ABL in the TFR for a long time had a decreased immunosuppressive Treg cells, while analysis using late recurrence increased Treg cells prior to recurrence (figure). These suggest the presence of cases in which early recurrence is suppressed by host immune responses.
Conclusion: Although the loss of function mutations such as BCR::ABL Ins35bp was previously thought to occur after TKI administration as a cause of TKI resistance, this analysis reveals that the clone is included from the ND before TKI treatment. Examining the BCR::ABL Ins35bp clone at ND may be possible to predict the possibility of recurrence after TFR. Host immune capacity, as assessed by the kinetics of Treg after TKI discontinuation, may contribute to prolonged TFR. Thus Treg monitoring may be important in predicting molecular recurrence, especially in BCR::ABL Ins35bp cases.
Disclosures
Takahashi:Astellas pharma: Other: Commissioned research and joint research , Research Funding; Asahi-Kasei: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Mochida Pharma: Research Funding; Otsuka Pharmacuetical: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal