Background: Infections in patients with multiple myeloma are a frequent cause of morbidity and mortality. Intravenous immunoglobulin (IVIG) replacement is a preventative strategy for infection risk reduction. The NCCN guidelines recommend IVIG therapy should be considered in the setting of recurrent serious infection and/or hypogammaglobinemia. The IMWG suggests a targeted use, limiting replacement to patients with IgG concentrations less than 400mg/dl and who have serious recurrent encapsulated bacterial infections. In this study, we sought to describe patterns of IVIG use prior to and during the COVID-19 epidemic.
Methods: In this cross-section study, we sought to describe real-world patterns of outpatient IVIG use before and during the COVID-19 pandemic using the IBM MarketScan Commercial Claims and Encounters (CCAE) and Medicare Supplemental and Coordination of Benefits (COB, a.k.a. MDCR) databases. MarketScan is an administrative database of diagnostic and treatment data for more than 30 million commercially insured individuals in the United States. Patients included in this study had private insurance or were enrolled in a Medicare advantage plan. IVIG administration data was grouped by patient age, sex, geographic region, and metropolitan area defined by the Census Metropolitan Statistical Area,. Pre-pandemic time period is defined as the time prior to March 2020.
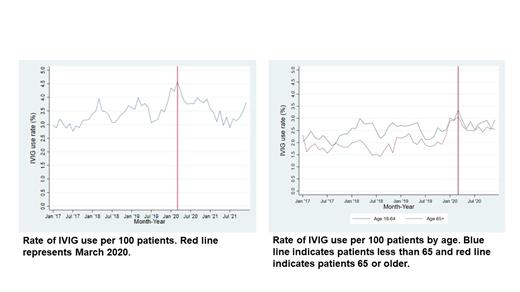
Results: Our study showed changes in patterns of IVIG use with respect to patient age, sex, geographic region, and location of care delivery. A total of 12,893 patients were identified with multiple myeloma during this study period. Patients greater than age 65 made up 31.3% of the cohort. Location of IVIG administration was outpatient hospital (32.6%), Office (40.2%), Patient home (14.9%) and other (0.9%). Pre-pandemic IVIG usage rate in this cohort was 3.37 IVIG administrations per 100 patients studied compared to post-pandemic usage rate increased to 3.6 per 100 patients (p=0.038). A significant increase in utilization is noted by age with usage rate in patients over age 65 increasing from 1.96 to 2.54 (p<0.001). Prior to the pandemic, usage rate for males was 2.2 and increased to 2.51 after the pandemic (p<0.001). For females, usage rate also increased from 2.42 to 2.58 (p=0.07). Changes in utilization also occurred regionally with increased use in the Northeast and West. Usage rates in the Northeast prior to the pandemic were 2.37 compared to 3.28 IVIG administration (p<0.001). Similarly, pre-pandemic utilization rates in the West were 2.55 and increased to 3.21 (p<0.001). Usage rates were stable in the North Central region, only increasing from 2.09 to 2.15 (p=0.54). Interestingly, usage rates decreased slightly in the Southern region from 2.32 to 2.24 post pandemic (p=0.38). Metropolitan service areas saw a slight increase in utilization from 2.41 to 2.56 (p=0.06). However, non-metropolitan service areas had an increase from 2.1 to 2.56 (p<0.001).
Conclusions: This study describes the variation in outpatient IVIG use by age, geographic region, sex and location of treatment before and during the COVID-19 pandemic. During the COVID-19 pandemic, there was significant uncertainty regarding optimal infection risk mitigation strategies for patients with multiple myeloma. In this real-world review of clinical practice patterns, variations in outpatient use of IVIG can be seen to vary most based on geographic region and patient age. Statistically significant increases are also seen in males and in non-metropolitan areas. Patients in regions impacted earliest and hardest by increase in COVID-19 infections saw the greatest increase in the use of IVIG while other regions saw stable or decreased utilization. Additionally, patients over age 65, who were at greatest risk for negative outcomes of COVID-19 infections saw the greatest increase in utilization compared to pre-pandemic rates. In the post pandemic time period, patients over age 65 continued to have statistically significant increased rates of utilization while patients less than age 65 also increased rates of utilization that was not statistically significantly increased. This study is limited by lack of clinical information including COVID 19 infection status and concomitant myeloma therapy. The variation in utilization of IVIG illustrates the need for prospective studies to guide its use.
OffLabel Disclosure:
Mckay:BMS: Consultancy. Bhutani:Janssen Research & Development: Research Funding; Bristol-Myers Squibb/Celgene: Research Funding; Amgen: Research Funding; Adaptive Biotechnologies: Research Funding; Takeda: Research Funding. Paul:Janssen: Membership on an entity's Board of Directors or advisory committees. Voorhees:Regeneron: Consultancy; Nervianos Medical Sciences: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Novartis: Consultancy; GSK: Consultancy, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Data Safety and Monitoring; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hurd:BMS/Celgene: Current equity holder in publicly-traded company.
IVIG is frequently used for infection prevention in patients with multiple myeloma. This study explores its use before and during the COVID 19 epidemic in the US. The data presented is aggregated data for use of IVIG as a billing code, not for a specific brand of IVIG.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal