Background: The treatment of newly diagnosed multiple myeloma (NDMM) in elderly patients is challenging due to various problems. To address these challenges, the European Myeloma Network (EMN) recommends conducting a frailty assessment before NDMM treatment in elderly patients. If the assessment identifies an intermediate fit or frail group, dosage reduction is recommended to avoid excessive treatment. However, as frailty status improves, there may be a risk of insufficient treatment leading to poor outcomes. In order to explore the optimal treatment approach for elderly NDMM patients in real-world settings, a retrospective analysis was conducted on the effectiveness of dynamically adjusting treatments based on frailty assessment in intermediate-fit and frail groups of elderly NDMM patients.
Methods: Patients who were initially diagnosed with multiple myeloma and assessed as intermediate fit or frail patients by IMWG-GA frailty assessment at the Department of Hematology, Affiliated Hospital of Hebei University from January 1, 2018 to April 1, 2023 were included in this study.The initial treatment regimen for all patients in this group consisted of a combination of Bortezomib with dexamethasone (BD) or Ixazomib with dexamethasone (ID) .Frailty assessment and efficacy evaluation were conducted every 2-3 courses during the treatment period. In the frail group, treatment in those frailty status improved was adjusted based on prognosis stratification by adding a third drug to high-risk R-ISS patients and subsequently adjusting it according to efficacy evaluation; additionally, a third drug was added to patients who did not reach partial response (<PR). For the intermediate fit group, sequential addition of a third drug occurred during courses 2-3.The options for the third drug included cyclophosphamide, lenalidomide, and thalidomide,and common regimens used were ICD, IRD, VRD, VCD, and VTD.The response rate, discontinuation rate, early death, and adverse drug reactions were observed.
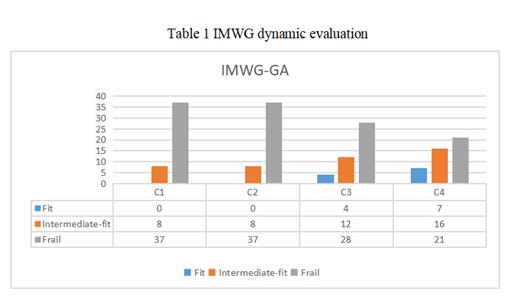
Results: A total of 45 patients with newly diagnosed NDMM were included in this study. Among them, 37 patients were assessed as frail .24 /45 patients were treated with the BD regimen and 21/45 patients with the ID regimen. During treatment, dynamic frailty assessment showed significant improvement in frail status during courses 3-4 (see Table 1). Following dynamically adjusted principles, additional drugs were administered to certain patients: 24 /37 frail patients received a third drug; among the intermediate fit patients, all received a third drug. The best ORR achieved throughout the study was at an impressive rate of 82.2% (37/45), with a corresponding best efficacy above VGPR seen in 51.1% (23/45) of cases. Twenty-one patients experienced disease progression for various reasons during treatment, with a median PFS of 14.0 (3.0-96.0) months. During the follow-up period, a total of 17 patients died, while none died within 6 months. The median overall survival (OS) was found to be 18.0 months (ranging from 3.0 to 115.5 months). Safety evaluation was conducted on all 45 patients within the first year and they tolerated the treatment well. Most adverse reactions reported were of grade 1-2 according to CTCAE version 5.0.
Conclusion: This study is a retrospective study that the initial treatment involved administering a low-intensity regimen consisting of either BD or ID two-drug combination. Serious adverse reactions, drug withdrawal, and early death decreased in both groups. The treatment intensity was increased for high-risk patients or those with poor efficacy in the frail group after the improvement of frailty status. This new therapeutic strategy which integrates dynamic changes of frailty status, cytogenetic risk factors, efficacy evaluation and dynamic adjustment of treatment, has shown improved efficacy for patients with two different levels of frailty. Additionally, it has been found to reduce early mortality rates. These promising results warrant further investigation through prospective studies.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal