Background: Multiple myeloma (MM) is a fatal, malignant disease of plasma cells characterized by an abnormal proliferation of monoclonal antibodies. China is estimated to have 22,450 newly diagnosed (ND) cases of MM and 17,360 related deaths in 2022. Emerging novel therapies have significantly improved survival in MM, and treatment guidelines have been updated to reflect these advances. Ninlaro® (ixazomib) is the first oral proteasome inhibitor (PI) approved by the US FDA in 2015 and by China's National Medical Products Administration in 2018 (conditionally) for MM patients receiving ≥1 prior therapy owing to its convincing efficacy and well-tolerated safety profile as seen in the landmark Phase III TOURMALINE-MM1 trial and the China Continuation Study. Novel drugs such as PIs, immunomodulatory drugs (IMDs), and anti-CD-38 monoclonal antibodies are the core component of MM treatment and have improved patient outcomes considerably. However, many patients remain refractory to these drugs (triple-class refractory, TCR) and have poor clinical outcomes. There is a lack of real-world evidence characterizing treatment patterns and outcomes of MM patients receiving ixazomib and TCR-MM patients, in China. The objective of this study is to bridge this data gap by providing vital information on the treatment patterns and outcomes, and healthcare resource utilization in Chinese MM patients receiving ixazomib and TCR-MM patients in real-world clinical practice.
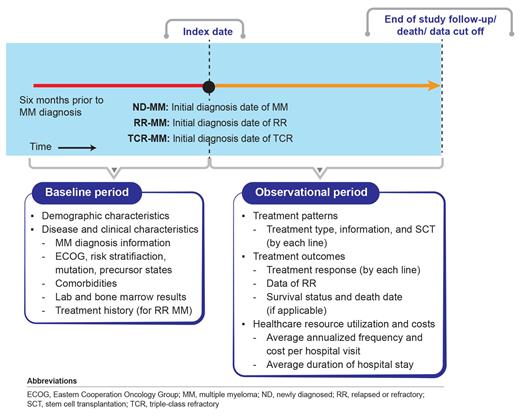
Study Design and Methods: This study is an ongoing, single-centre, non-interventional, retrospective database analysis study of MM patients identified from the Chinese National Longitudinal Cohort of Haematologic Diseases (NICHE)-MM registry, a historical and ongoing longitudinal data registry of the Institute of Haematology and Blood Diseases Hospital (IHBDH), and representing the top-tier MM care in China. The patients will be divided into two groups. Patients (aged ≥18 years) with a confirmed MM diagnosis receiving all ixazomib-based regimen as 2nd-line and above therapy (RR-MM), or as a 1st-line therapy (ND-MM) will form group one and TCR-MM patients' refractory to at least one IMiD (lenalidomide, pomalidomide or thalidomide), one PI (bortezomib, ixazomib, or carfilzomib) and one anti-CD-38 monoclonal antibody (daratumumab and isatuximab) will form group two. TCR-MM patients receiving ixazomib will be part of both groups. Patients enrolled in clinical trials will be excluded. The data for analysis will be collected from the ixazomib launch date in China (April 12, 2018) and/or the index date of the first patient enrolled in ixazomib Named Patient Program to September 2023 (tentative). Since 2000 till January 2023, the NICHE-MM registry has enrolled 3,329 MM patients of whom 156 patients have received ixazomib. This is the preliminary sample count and the final sample size will be calculated during data cleaning and analysis phases.
The primary objective of this study will be to determine 1) treatment patterns and responses by lines of treatments and 2) time-to-event outcomes. The treatment patterns will include treatment name, dosage, type (induction, consolidation, or maintenance), number of cycles, duration and severe adverse event causing hospitalization. The treatment responses will include complete response (CR), stringent CR, partial response (PR), very good PR, minimal response, stable disease, and progressive disease as defined by the International Myeloma Working Group. The overall response rate of the last line of treatment for both study groups will be calculated as composite endpoint. Time-to-event outcomes will include progression-free survival, overall survival, duration of therapy, and time to next line of treatment in ND/RR-MM and TCR-MM patient groups. Secondary objectives will include assessment of patients' demographic, disease and clinical characteristics. Depending on data availability, the exploratory objective will be to determine the healthcare resource utilization and costs incurred by patients in both groups. Subgroup analysis between ND-MM vs. RR-MM or RR-MM patients receiving ixazomib in 2nd-line setting vs. TCR-MM patients receiving ixazomib in >3rd-line setting is also planned. Descriptive statistics will be used for the analyses.
Our findings will also help clinicians in making strategic decision about more apt positioning of ixazomib in the treatment algorithm of MM.
OffLabel Disclosure:
Li:Takeda (China) International Trading Co., Ltd.: Current Employment. Liu:Takeda (China) International Trading Co., Ltd.: Current Employment.
In combination with lenalidomide and dexamethasone, it is used to treat adult patients with multiple myeloma who have received at least one previous treatment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal