Background:
Hematopoietic stem and progenitor cell (HSPC) transplant is an essential treatment for a variety of blood disorders and malignancies, and more recently, for gene editing therapies such as sickle cell anemia. A key step in this procedure is the effective mobilization of a large quantity of primitive donor HSPCs to ensure a durable and long lasting engraftment capable of developing into all blood cell lineages. The most commonly used regimen for donor mobilization is a 5-day course of Granulocyte Colony Stimulating Factor (G-CSF). However, G-CSF does not effectively mobilize a sufficient number or quality of stem cells in all donors, can have unwanted side effects, and is contraindicated in sickle cell disease. Therefore, it is desirable to identify an alternative treatment that is safe, rapid and cost effective. The cell surface integrin receptor VLA-4 (a4b1) and the chemokine receptor CXCR4 are essential for HSPC homing and retention in the bone marrow. Inhibition of these receptors rapidly mobilizes HSPCs within an hour, as opposed to the multi-day process using G-CSF. Small molecule inhibitors of VLA-4 have been reported previously, but most have poor pharmacokinetic properties and/or weak inhibition of VLA4. These inhibitors rapidly mobilize HSPCs into the peripheral blood of mice after a single s.c. injection, but their effect is short lived, yielding an inadequate number of HSPCs necessary for stem cell transplantation or for gene therapy. Therefore, VLA4 inhibitors with improved pharmacokinetic properties imparting extended and greater HSPC mobilization after a single dose is desirable.
Methods:
We designed and synthesized multiple novel VLA-4 inhibitor molecules and tested their potency using soluble VCAM-1 binding assays. Inhibitors determined to be most potent with improved aqueous solubility were then tested in vivo in DBA mice for extended HSPC mobilization beyond 4 hours after a single SC injection, alone and in combination with the CXCR4 inhibitors.
Results:
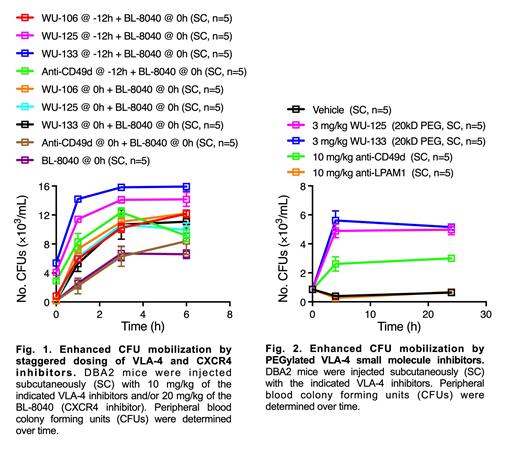
We developed two main core molecules that each incorporate key VLA4 receptor binding motifs critical for potent inhibition of VLA4 binding to VCAM-1. Our first generation of inhibitors were quite potent and rapidly mobilized HSPCs, but their effect was short lived and yielded insufficient numbers of HSPCs for gene editing and engraftment studies. Most had poor solubility and very short plasma half-lives after s.c. injection in mice. We designed later generation analogues that strategically exploited a region of each optimized core, allowing for covalent addition of polyethylene glycol (PEG) chains of increasing length without decreasing potency. We determined that a minimum PEG length of 24 PEG units was required for extended HSPC mobilization in mice. WU-106, one of our VLA-4 inhibitors containing 24 PEG units, exhibited excellent aqueous solubility, longer plasma half-life after s.c. dosing, and mobilized 2-fold more murine HSPCs for a longer time than previously published best-in-class VLA-4 inhibitors or our first generation VLA4 inhibitor leads. Importantly, a synergistic effect on HSPC mobilization was achieved when WU-106 was co-administered with CXCR4 inhibitors (Plerixafor or BL-8040). Further optimization by the addition of longer PEG chains, even up to 40KD, retained sub nM potency and extended mobilization beyond 24 hours after a single injection. Our 20kD PEG analogues attached to our two different core molecules (WU-125 and WU-133) allowed for dosing of the drug in the evening, with maximum mobilization still present the next morning, thereby enabling HSPC collection to initiate soon (2-4 hours) after CXCR4 inhibitor dosing in the morning. The quantity of HSPCs obtained using this staggered dosing regimen was greater than simultaneous administration of the VLA-4 and CXCR4 inhibitors ( Fig. 1). Furthermore, our novel, long-acting PEG analogues demonstrated superior HSPC mobilization compared to an alpha 4-targeted antibody (anti-CD49d) in mice ( Fig.2).
Conclusion:
We have developed novel, potent PEGylated small molecule VLA-4 inhibitors that have excellent aqueous solubility and pharmacokinetics. The kinetics and magnitude of HSPC mobilization by our PEGylated inhibitors are superior to other previously described VLA-4 inhibitors and an antibody to alpha-4(anti-CD49d).
Disclosures
DiPersio:Magenta: Other: Ownership Investment, Patents & Royalties; Incyte: Consultancy, Research Funding; RiverVest Venture Partners: Membership on an entity's Board of Directors or advisory committees; BiolineRx Ltd: Consultancy, Research Funding; Macrogenics: Consultancy, Research Funding; Washington University: Current Employment; Vertex: Consultancy; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; WUGEN: Other: Ownership Investment, Patents & Royalties; Amphivena Therapeutics: Research Funding; Sun Pharma Ltd.: Membership on an entity's Board of Directors or advisory committees; NeoImmune Tech: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal