Background: Anti-thymocyte globulin (ATG) serotherapy for graft-versus-host disease (GVHD) prophylaxis in allogeneic hematopoietic cell transplantation (HCT) is frequently associated with infusional side-effects (ISEs) including fever, tachycardia, respiratory distress, and hypotension/shock. Although this constellation closely resembles cytokine release syndrome, the mechanism behind ATG ISEs is poorly understood. The aim of this study was to characterize the frequency and severity of ATG ISEs, and determine the association of ISEs with cytokine levels and clinical outcomes.
Methods: This study included 118 adult HCT recipients receiving myeloablative conditioning including fludarabine, busulfan, low-dose total body irradiation, and 4.5mg/kg ATG (0.5mg/kg on day -2 and 2mg/kg on days -1 and 0). Patient electronic medical records were retrospectively reviewed for clinical data collection. ISE were classified as thermal (fever/rigors), respiratory (tachypnea/hypoxemia/increased O 2 supplementation), tachycardia, or circulatory (hypotension/fluid resuscitation requirement). ISEs were further characterized into mild or severe (e.g., mild tachycardia = 105-120bpm, severe ≥120bpm). Serum levels of 31 cytokines were determined pre- and post-1 st ATG infusion in all patients (and up to 13 timepoints in select patients) using Luminex. Cox and Fine-Gray regression were used to determine the association of ISEs with clinical outcomes including grade 2-4 acute GVHD (aGVHD), moderate-severe chronic GVHD (cGVHD), cumulative incidence of relapse (CIR), and overall survival (OS).
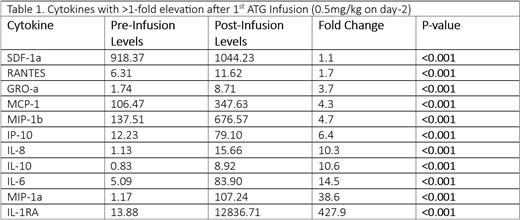
Results: At least one ISE occurred in 74% (87/118) of patients, with a maximum severity of mild in 56 (47%) and severe in 31 (26%). Patients experienced an average of 1.4 ISEs (median 1), most commonly tachycardia (57%) and thermal (50%), while respiratory (26%) and circulatory (7%) ISEs were less frequent. ISE frequency was 62%, 60%, and 35% after the 1 st-3 rd ATG infusions, respectively, with the median time from infusion to first ISE being ~4.5 hours. Compared to pre-infusion levels, there was a >1-fold change in 11/34 cytokines (all P<0.001) [Table 1]. Of greatest interest was IL-6, which demonstrated a progressive increase in median levels between patients with no vs. mild vs. severe ISEs (P<0.05). Severe ISEs were associated with a lower incidence of grade 2-4 aGVHD (SHR=0.12, P=0.032), but not with any other outcomes. Patient age, sex, primary disease, or pre-infusion peripheral blood cell counts (leukocytes, lymphocytes, neutrophils, and monocytes) did not appear to influence ISE frequency or severity.
Conclusion: ISEs occur in the majority of ATG recipients, with one-fourth experiencing severe ISEs. The concomitant rise in serum cytokine levels (particularly IL-6) suggests that anti-cytokine antibodies (e.g., tocilizumab) may assist in the prevention or treatment of ISEs, however, the impact this may have on outcomes is unclear and warrants investigation in upcoming clinical studies.
Disclosures
Storek:Sanofi-Pasteur: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal