Background: Varnimcabtagene autoleucel (IMN-003A) is an anti-CD19 CAR-T cell immunotherapy for patients (pts) with relapsed refractory B cell malignancies (RR BCM) in first-in-India Industry Phase 2 IMAGINE study, administered in a fractionated (10%/30%/60%) infusion protocol.
Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) are commonly associated adverse events with CAR-T cell therapy. The modified Endothelial Activation and Stress Index (mEASIX) score [lactate dehydrogenase (LDH) (U/L) × CRP (mg/dL) / platelets (PLT) (10 9 cells/L)] is a prognostic predictor of outcomes for patients in post-allogeneic transplant setting. Elevated LDH and low PLT levels have been associated with severe ICANS, and high IL-6 level is a recognized marker for CRS. Immune Effector Cell Associated Hemato-Toxicity (ICAHT) represents the most common toxicity after CAR-T cell therapy. CAR-HEMATOTOX score is used to risk stratify patients for cytopenia.
This abstract reviews the mEASIX score and ICAHT for pts in the IMAGINE study, prognostic value of mEASIX for CRS, ICANS and responses, and correlation of ICAHT score with outcomes.
Methods: mEASIX scores were calculated for each pt at lymphodepletion (LD), D0, D+1 and D+4. CRS and ICANS were graded as per American Society for Transplantation and Cellular Therapy (ASTCT) criteria. Pts were monitored for ICAHT post CAR-T infusion and risk stratified using CAR-HEMATOTOX score. ICAHT was graded using CTCAE v5.0. Appropriate statistical tools were used for analysis.
Results: Twenty-four RR BCM pts (12 B-ALL; 12 B-NHL) were treated with var-cel in this study. Overall response rate (ORR) was 91.7% (22/24) at D+28 (B-ALL 91.7%; B-NHL 91.7%) and 80.9% (17/21) at D+90 (B-ALL 80%; B-NHL 81.8%).
AESIs reported were CRS (Grade [G] 1 62.5%; G3+ 4.2%; overall 66.7%); ICANS (G1 4.2%; G3+ 0%; overall 4.2%); neutropenia (G3+ 91.7%; overall 100%); anemia (G3+ 29.2%; overall 95.8%); and thrombocytopenia (G3+ 20.8%; overall 91.7%). CRS median onset was D+5 and duration 3 days. No G3+ ICANS was reported. Treatment related mortality was 4.2% (n=1/24, CRS); 3 pts died of disease progression.
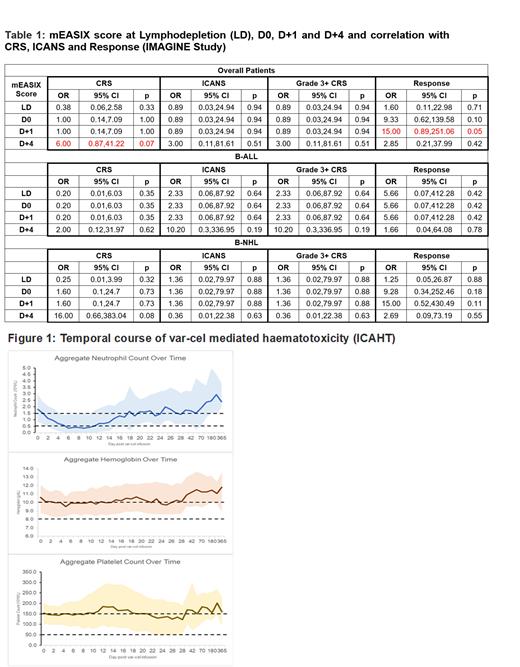
The mEASIX score at LD, D0, D+1 and D+4 and its correlation with CRS, ICANS and responses is shown in Table 1. At D+1, mEASIX score <1 was predictive for responses (entire cohort, p=0.05; OR 15.0). At D+4, mEASIX score >1 was nearing statistical significance (p=0.07) for CRS (all grades; OR 6.0). However, mEASIX score at other timepoints was not predictive for CRS, ICANS or responses.
The incidence of ICAHT was 100% (all grades; n=24), 100% neutropenia (G3+ 91.7%); 95.8% (n=23) anemia (G3+ 29.2%) and 91.7% (n=22) thrombocytopenia (G3+ 20.8%). The median time (range) to resolution of anemia, neutropenia and thrombocytopenia was 90 (11-270), 17.5 (1-181) and 9 (1-181) days respectively. Classical, biphasic and prolonged cytopenia pattern was seen for Neutropenia in 6, 2 and 16 pts respectively; for Anemia in 2, 0 and 21 patients respectively and for Thrombocytopenia in 4, 1 and 17 patients respectively. Overall, all patients, except two, were pancytopenic during their treatment course. Figure 1 shows the temporal course of var-cel mediated haematotoxicity (ICAHT). Two pts (8.3%) each received red cells, platelets, G-CSF and romiplostim; majority did not need supportive treatment.
CAR-HEMATOTOX score was low risk in 18 pts and high risk in 6 pts. There was no statistical correlation between low risk (18 pts) and high risk (6 pts) with responses (17 pts, p=0.92) or with relapse / disease progression (9 pts, p=0.21).
Conclusions: This is the first-in-India industry study evaluating mEASIX and ICAHT in patients treated with CAR-T cell therapy. mEASIX score <1 was predictive of responses at D+1 and score >1 at D+4 was mildly predictive of CRS. All patients developed ICAHT but only a small number of patients needed supportive treatment. Overall, var-cel was well tolerated with manageable cytopenia. The CAR-HEMATOTOX score was not predictive of relapse / disease progression in the IMAGINE study.
mEASIX score may be used as an early predictor of CRS for permissive use of Tocilizumab before onset of severe symptoms. The ICAHT profile was heterogeneous, probably reflective of hematopoietic reserve, lines of treatment and downstream immune responses. The predictive role of mEASIX score and ICAHT profile in a real-world setting needs further study.
Disclosures
BR:Immuneel Therapeutics Private Limited: Current Employment. Arasu:Immuneel Therapeutics Private Limited: Current Employment. Elluru:Immuneel Therapeutics Private Limited: Current Employment. Akheel:Immuneel Therapeutics Private Limited: Current Employment. Kamat:Immuneel Therapeutics Private Limited: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal