Haploidentical peripheral blood stem cell transplantation with post-transplant cyclophosphamide (Haplo-PTCy) is the most common transplantation modality in low-and-middle-income countries (LMIC). Registry data has shown comparable outcomes when using haploidentical donors versus Matched Unrelated Donors (MUD) in patients with acute leukemias. In the Chilean Public Health System Haplo-PTCy was incorporated in 2016 for adults up to 60 years old.
We analyzed all adult patients who received an Haplo-PTCy at Hospital del Salvador, in a prospective registry study between 2016 and 2021. The primary outcome was overall survival (OS). Secondary outcomes were event-free survival (EFS), cumulative incidence of relapse, and incidence of grade II-IV acute graft-versus-host disease (aGVHD) at day +100. Overall survival and EFS were estimated using the Kaplan-Meier method. The cumulative incidence of relapse was calculated using relapse as the primary event and death without relapse as a competing event. Acute GVHD is shown as absolute incidence at day +100. All statistical analyses were performed with R. This study was done in compliance with the Helsinki Declaration and was approved by the Institutional Review Board.
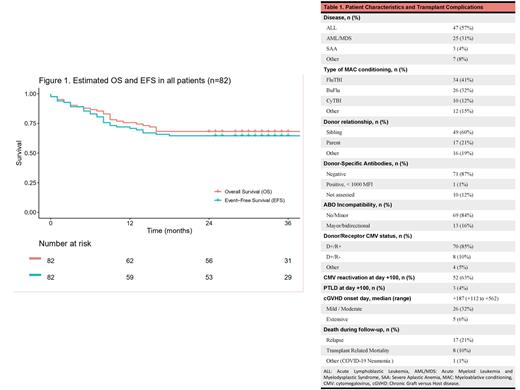
Eighty-five Haplo-PTCy were performed in 82 patients (Table 1). Three-second Haplo-PTCy were excluded from this analysis. The median age was 25 years (range, 15-51), and 65% were male. Ninety-four percent of patients had a neoplastic disease (77/82), and the most common diagnosis was acute lymphoblastic leukemia (57%). Forty-seven percent proceeded to transplant in the first complete response. Most of the patients (76%) presented a low hematopoietic cell transplantation (HCT)-specific comorbidity index. Conditioning was mostly myeloablative (96%). The median dose of CD34+ cells infused was 8.1 x 106/kg (range, 2.4-10.0). No patient needed a desensitization regimen. Cytokine release syndrome (CRS) was common (86%) but no grade 3 CRS was observed. Primary graft failure was observed in one patient (1.2%). Poor graft function was observed in 11 patients (13%). Five patients (6.1%) died before engraftment. The incidence of grade II-IV aGVHD at day +100 was 29% (grade IV was not observed). With a median follow-up of 33 months (range 0-84), the estimated 3-year OS and EFS were 68.3% (95% CI 59-79%) and 64.6% (95% CI 55-76%), respectively (Figure 1). In patients with neoplastic disease (n=77), the 3-year cumulative incidence of relapse was 23% (95% CI 15-33%).
Haplo-PTCy is feasible in LMIC. This cohort of cases, mostly young patients with neoplastic disease, shows encouraging survival with an acceptable aGVHD and relapse incidence.
Disclosures
Undurraga:AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; Novartis: Speakers Bureau; Pfizer: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal