Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the curative treatment for acute Myeloid Leukemia (AML) patients. When there is no HLA-identical sibling or unrelated donor, alternative donors such as 1 allele-mismatched unrelated donors (1-MMUD) or haploidentical donors (HID) may be considered. While there have been reports that the outcomes of these two donors are comparable with those of HLA-matched unrelated donors, there is limited data directly comparing the outcomes between 1-MMUD and HID transplantation. Therefore, we conducted an analysis of the outcomes of allo-HSCT from 1-MMUD and HID in AML patients.
This study is a retrospective cohort study at a single institution, including patients with AML who underwent first allo-HSCT in complete remission (CR) or CR with incomplete hematologic recovery between 2002 and 2022, from 1-MMUD or HID. For HID transplantation, patients were administered fludarabine 30mg/BSA for 4 days, intravenous busulfan 3.2mg/kg for 2 days, and depending on the condition, they received either total body irradiation (TBI) at 400cGy or fractionated TBI at 800cGy (400cGy for 2 days). Graft-versus-host disease (GVHD) prophylaxis for HID transplantation consisted of antithymocyte globulin total 5.0mg/kg, methotrexate 5mg/BSA 3 or 4 times, and calcineurin inhibitors. In 1-MMUD transplantation, various myeloablative or reduced-intensity conditioning regimens were used. All patients received T-cell replete peripheral blood stem cells.
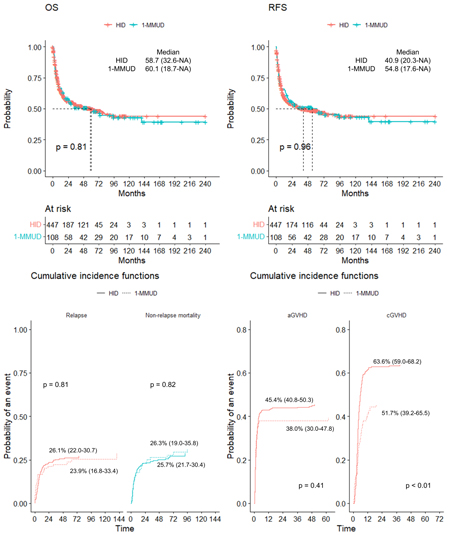
A total of 108 patients who underwent 1-MMUD transplantation and 447 patients who underwent HID transplantation were included. The 1-MMUD group showed a tendency to be younger (median 42 years vs. 51 years, Wilcox rank-sum test p < 0.01) and had a lower proportion of ABO-matched transplantations (28.7% vs. 51.7%, Chi-square test p < 0.01). Other patient characteristics were similar between the two groups. In terms of survival, the median OS in the 1-MMUD group was 60.1 months, and in the HID group was 58.7 months, showing no significant difference between the two groups (Log rank test p = 0.81). Similarly, the median RFS was 54.8 months in the 1-MMUD group and 40.9 months in the HID group, with no statistically significant difference (Log rank test p = 0.96). The cumulative incidence of relapse and NRM at 60 months after allo-HSCT showed no significant difference between the two groups, with 26.1% and 25.7% for relapse and 23.9% and 26.3% for NRM in the 1-MMUD and HID groups, respectively (Gray's test p for relapse: 0.81, p for NRM: 0.82). Although the cumulative incidence of grade 2 or higher acute GVHD did not show a significant difference between the two groups (38.0% in 1-MMUD, 45.4% in HID, Gray's test p = 0.41), the incidence of chronic GVHD was significantly higher in the HID transplantation group (63.6%) compared to the 1-MMUD transplantation group (52.0%) (Gray's test p < 0.01). Finally, in a multivariate Cox model
In conclusion, both 1-MMUD and HID can be considered equivalent alternative donors, and caution should be exercised regarding the occurrence of chronic GVHD in HID transplantation. Further comparisons between the two donors, including in different settings such as post-transplantation cyclophosphamide, warranted.
Disclosures
Lee:Alexion, AstraZeneca Rare Disease: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Achillion: Research Funding; AlloVir: Consultancy; Arrowhead: Consultancy; Kira: Consultancy; Samsung: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal