Introduction: Nanopore sequencing (NS), a third generation long-read sequencing technology, is increasingly being used to study cancer genomes. NS has many advantages over next-generation sequencing, including the capability to simultaneously extract base sequence as well as base modifications in the same run without any further library modification. Notably, its portability and ability to provide real-time data make it a potentially powerful tool for clinical applications. This study explores the use of NS to directly sequence native cell free DNA (cfDNA) from patients with diffuse-large B-cell lymphoma (DLBCL), the most common type of non-Hodgkin lymphoma.
Methods: For this exploratory study, 20 patients with either a new diagnosis or relapsed DLBCL were enrolled. We adapted NS methods for cfDNA and sequenced cfDNA before each chemotherapy cycle using both an Illumina platform (as a control) and the Nanopore platform. For differential methylation, we also sequenced plasma cfDNA from five healthy donors on the Nanopore.
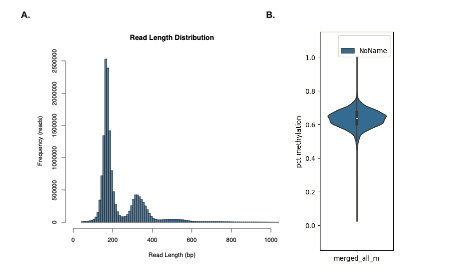
Results: Our findings demonstrate that we can directly sequence cfDNA on the Nanopore platform, obtaining a whole genome coverage (average 1.2x) with deeper coverage in some regions and also observe ~60% global DNA methylation level in CG context. From this data we are now analyzing regions that are differentially methylated (DMRs) between normal and DLBCL, determining SNV/Indel profiles, and comparing copy number variants (CNVs). These results will be available and presented at the time of the meeting.
Conclusions: NS is capable of detecting cfDNA and measuring methylation levels, offering a promising and potentially clinically applicable new approach for the study of cfDNA.
Future directions: As we continue this research, our vision is to leverage the portability and real-time data capabilities of NS for clinical applications. By simultaneously combining multiple genomic features with machine learning, we aim to develop a method for measuring lymphoma cfDNA-based MRD in DLBCL patients to be used in clinical settings.
Disclosures
Gribben:AbbVie: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Research Funding; Janssen Pharmaceuticals, Inc: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Kite, A Gilead Company: Consultancy, Speakers Bureau; Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal