Background
Managing sickle cell disease (SCD) and its associated complications requires specialized knowledge often limited in primary and emergency care settings. Despite the availability of guideline recommendations and an expanding therapeutic landscape, hematologists experience challenges in managing sickle cell pain and implementing multidisciplinary care coordination that improves morbidity, overall disease management, and quality of life. To improve SCD care, we conducted a quality improvement (QI) study, bringing together healthcare professionals (HCPs) from established SCD centers to assess current practice patterns and develop strategies to overcome barriers to acute pain management and transitions of care for patients with SCD.
Methods
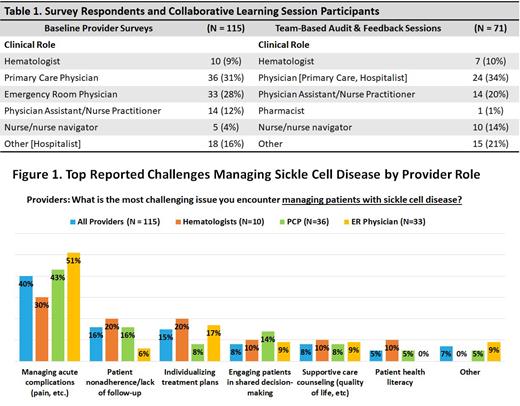
This QI initiative is comprised of baseline surveys (n = 115), pre- and post-surveys (n = 71) from the small-group, team-based audit-feedback (AF) sessions (Table 1), and 90-day follow-up surveys of HCPs from the four enrolled centers. Survey questions were designed to assess knowledge, confidence, and experiences managing acute pain, transitioning care, and keeping up with the latest clinical evidence and guideline recommendations for SCD. Care teams from each clinic, along with a nationally renowned expert SCD physician, participated in audit and feedback sessions to (a) assess system-specific practice gaps identified via the provider surveys, (b) prioritize areas for improvement, and (c) develop action plans for addressing root causes.
Results
Baseline surveys results revealed providers' top challenge encountered in managing patients with SCD as managing acute complications (40%) (Figure 1). Gaps were observed in clinicians reporting high/very high confidence in their ability to adequately manage SCD patients' acute pain episodes (51%), align clinical practices with evidence-based SCD guidelines (44%), and coordinate transitions of care (33%). Furthermore, baseline surveys showed only 19% of providers reported they are very/extremely satisfied with their current process for transitioning patients, noting top challenges in patient's difficulty navigating the adult healthcare system (47%) and deficiency of providers caring for patients with SCD near me (26%).
Following participation in the AF sessions, clinicians indicated gains in high/very high confidence in their ability to adequately manage an acute pain episode for a patient with SCD (52% to 69%) and ability to coordinate transitions of care for patients with SCD (44% to 56%). During the AF sessions, Care teams set goals to improve provider education, appropriate acute care management of SCD and patient engagement. Action plans to achieve these goals include sharing the action plan with additional clinical team members (47%), collaborating among clinical teams to improve pain management plans for patients with SCD (39%) and improving patient education about SCD (31%).
In 90-day follow up surveys, 75% of clinics reported improvements in acute pain management and transitions of care for patients with SCD. Furthermore, the top 3 areas of most improvement at the time of follow-up were enhancing sickle cell education provided to patients (75%), supporting teenage and young adult patients in independently managing their own care (50%), and coordinating meetings with local adult sickle cell providers and teenage/young adult patients and families (50%).
Conclusions
Through this QI initiative, clinical teams identified barriers to optimal SCD care, creating and implementing specific action plans to increasing knowledge of SCD and evidence-based therapies, optimizing transitions of care and improving acute pain management. While meaningful confidence and action plan gains were demonstrated, these data underscore clinical practice gaps to address in future initiatives to support safe, effective, and evidence-based processes for managing SCD to prevent complications and optimize outcomes and quality of life.
Study Sponsor Statement
The study reported in this abstract was funded by educational grants from Vertex Pharmaceuticals Incorporated and Agios Pharmaceuticals, Inc. The grantors had no role in the study design, execution, analysis, or reporting.
Disclosures
Andemariam:NovoNordisk: Consultancy; PCORI: Research Funding; Pfizer: Research Funding; Sanofi Genzyme: Consultancy; Vertex: Consultancy; Global Blood Therapeutics: Consultancy, Research Funding; GSK: Consultancy; Bluebird: Consultancy; Forma Therapeutics: Consultancy, Research Funding; Emmaus: Consultancy; Connecticut Department of Public Health: Research Funding; Agios: Consultancy; American Society of Hematology: Research Funding; Novartis: Consultancy, Research Funding; HRSA: Research Funding; Hemanext: Consultancy, Research Funding; Afimmune: Consultancy; Accordant: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal