Background: The effectiveness of treatments in follicular lymphoma is evaluated by the reduction in the rate of recurrence. One of the methodological difficulties is related to the presence of competitive events such as death or second primary malignancies. The related cumulative incidence functions (CIF) are always estimated by using the non-parametric Aalen-Johansen estimator and the associated predictors by using the Fine and Gray approach or by considering a cause-specific Cox model. In the present study, we investigated if the use of a parametric mixture model would provide additional information on the interpretation of the impact of treatments in the management of follicular lymphoma.
Methods: We used the RELEVANCE study database (Morschhauser et al.NEJM 2018, JCO 2022) because of the prolonged follow-up of patients treated for follicular lymphoma. RELEVANCE study is a multicenter, phase 3 randomized clinical trial that evaluates rituximab lenalidomide (R 2) as compared to rituximab plus chemotherapy (R-chemo), in patients with previously untreated follicular lymphoma. All 1030 patients received a rituximab maintenance.
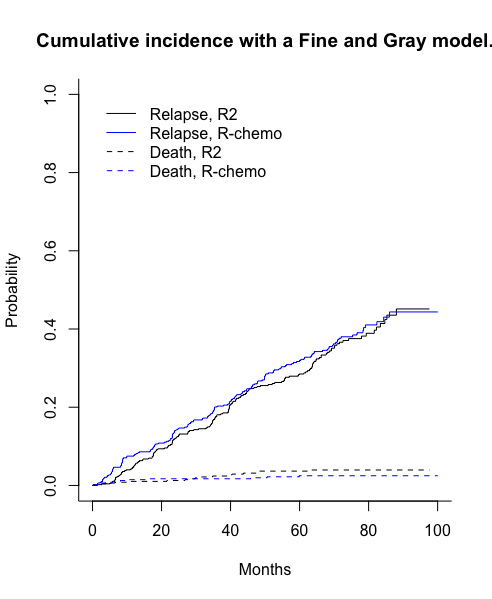
Results: With a median follow up of 72 months, median progression-free survival (PFS) was not reached in both groups (Kaplan-Meier estimator): the 6-years PFS was 60% and 59% for R 2 and R-chemo, respectively (Cox model, HR = 1.03 [IC95% CI, 0.84 to 1.27]). The CIF of relapse at 72 months was 41% and 39% in the R 2 and R-chemo, respectively (Aalen-Johansen estimator, Figure 1). We did not highlight any significant difference between the treatments in terms of the CIF of relapse (Fine and Gray model, HR = 0.938 [IC95% CI, 0.754 to 1.17], for the same prognostic time, the CIF of death was 2,5% and 2,9% in the R 2 and R-chemo, respectively).
We then performed the test with the mixture model, and confirmed there was no significant differences across groups at 72 months. Indeed, 64% and 96% of patients have not relapsed and died respectively.
However the mixture model approach allows for extrapolation, and thus we predicted the long-term CIF of relapse to be 67% if patients were followed-up 20 years across groups. The mixture model extrapolation calculated that 60% of relapse would occurr between 72 months and 240 months (20 years).
Conclusion: There was no difference at 72 months for survival across tests; but the mixture model, that allow extrapolation, suggested that a difference could be revealed with a greater follow-up.
We concluded that future studies of survival in FL will require longer follow-up. This comment appears of particular importance in the context of the forthcoming immunotherapies such as bispecific and CAR-T cells.
We can also propose for hematologic malignancies with a chronic evolution, FL, alternative primary outcomes not based on survival, allowing a shorter read out, such as the disability-adjusted life years (DALYs) or quality-adjusted life years (QALYs).
Disclosures
Guidez:Gilead/Kite: Honoraria; Astra-Zeneca: Honoraria; Incyte: Honoraria; Takeda: Honoraria. Gyan:Janssen: Consultancy, Honoraria; Abbvie: Honoraria; Astra Zeneca: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Fresenius Kabi: Research Funding; Gilead: Honoraria; Roche: Honoraria; Alexion: Honoraria; Chugai Pharma: Research Funding; Recordati: Consultancy. Deconinck:STEMLINE MENARINI: Consultancy; Immunogen Inc.: Honoraria; NOVARTIS: Research Funding; GILEAD KITE: Other: Hospitality, Research Funding. Johnson:Abbvie: Consultancy; Merck: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Gilead: Consultancy. Casanova:Takeda: Consultancy, Speakers Bureau; GSK: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; BeiGene: Consultancy; Sanofi: Speakers Bureau. Terui:Chugai Pharmaceutical Inc, Roche: Speakers Bureau; Symbio Pharmaceutical: Speakers Bureau; Eizai: Speakers Bureau; Bristl Myers Squibb: Speakers Bureau; Ono: Speakers Bureau. Morschhauser:Incyte: Other: Advisory Board; Novartis: Consultancy, Other: Advisory Board; Celgene: Other: Advisory Board; BMS: Consultancy, Other: Advisory Board; AbbVie: Consultancy, Other: Advisory Board; Janssen: Honoraria; Gilead: Consultancy, Other: Advisory Board; Roche: Consultancy, Honoraria, Other: Advisory Board; Epizyme: Other: Advisory Board; Genmab: Consultancy, Other: Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal