CAR T cell therapies have revolutionized the treatment of B cell malignancies. Ex vivo CAR manufacturing is complex, costly, and cumbersome, prompting efforts that develop shorter manufacturing and allogeneic or “universal donor” strategies. Conventional ex vivo CAR T cell therapy is not widely accessible and financial burdens to patients further hinder access to care. Furthermore, recent data show that CAR T product efficacy is correlated with reduced ex vivo manipulation of cells.
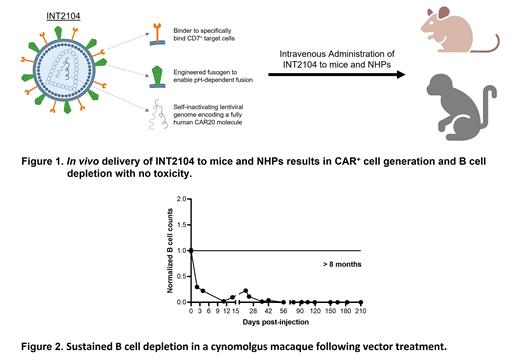
Here, we have designed a gene delivery system to transduce effector cells inside the body to generate autologous CAR cells, circumventing ex vivo cell manipulation, avoiding patient conditioning chemotherapy, and providing a low cost, “off-the-shelf” therapy. The product, INT2104, is an intravenously administered lentiviral vector encoding an anti-CD20 CAR transgene. INT2104 was rationally designed with an engineered fusogen and a novel binder to enable targeted transduction of CD7 + T and NK cells. The CAR20 molecule encoded by INT2104 is constructed of fully human domains, including a human and NHP cross-reactive anti-CD20 scFv, enabling preclinical studies in both humanized mouse and NHP models (Figure 1).
Prior to animal studies, INT2104 was extensively assessed in vitro for targeting specificity and the ability to generate functional effector cells. Incubating INT2104 with activated primary human PBMCs confirmed transduction of CD4 and CD8 T cells as well as NK cells. No B cell transduction was seen across a wide range of MOIs when INT2104 was used to inoculate a large panel of B cell tumor lines and primary PBMCs isolated from patients with B cell malignancies and healthy donors. INT2104-treated PBMCs cocultured with B cell tumor targets resulted in dose-dependent tumor cell killing.
In vivo evaluation of INT2104 has been conducted in three humanized mouse models. In CD34-engrafted NSG mice, delivery of INT2104 via tail vein injection resulted in B cell depletion within 7 days, with CAR + cells detectable in blood coincident with B cell ablation. In an established B cell tumor NSG model, INT2104 administration also resulted in B cell aplasia. In this tumor model, complete tumor ablation was seen in all treated mice across a 15-fold range in dosing, including a dose matching the proposed FIH dose in TU/kg. Protection from a re-challenge with tumor cells was also demonstrated. Finally, transgenic hIL15-expressing CD34-engrafted NSG mice were used to demonstrate in vivo generation of both CAR NK cells and CAR T cells in animals having B cell depletion.
In preclinical studies, 15 of 16 cynomolgus macaques receiving the CAR20 vector achieved at least a 75% reduction in circulating B cells following vector infusion. Some animals experienced a variable return of circulating B cells that was associated with immune responses to the CAR protein (anti-CAR responses). One animal (with a marginal immune response to the CAR) has shown complete and prolonged B cell depletion associated with persistence of the CAR transgene in peripheral blood and bone marrow (Figure 2).
Taken together, these data suggest that intravenous delivery of INT2104 will be both safe and effective, providing a faster, cheaper, and more accessible option for treatment of B cell malignancies. Ongoing efforts include a GLP Toxicology study to enable IND submission in 2Q2024.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal