Background Sickle cell anemia (SCA) complications such as frequent pain, chronic anemia, and acute and chronic organ damage lead to reduced physical functioning and cardiopulmonary fitness in affected individuals. Limited data support the safety of maximal cardiopulmonary exercise testing (CPET) in SCA, yet the safety of submaximal, longer-duration exercise at moderate to high intensity in SCA is unclear. The Sickle Cell Pro-Inflammatory Response to Interval Testing Study (SPRINTS) was a multicenter, prospective study that evaluated the impact of longer, submaximal exercise, which mimics the physical activity patterns of children better than CPET, on the acute inflammatory response and airway dynamics in children and young adults with SCA. In this analysis, we defined the effect of moderate to vigorous exercise on forced expiratory volume in 1 second (FEV1) and acute bronchoconstriction. We hypothesized the risk of exercise-induced bronchoconstriction (EIB) in children with SCA is greater than in controls.
Methods SPRINTS was approved by the Lurie Children's central Institutional Review Board. Subjects 10-21 years old with hemoglobin (Hb) SS or S/β0 thalassemia and race-matched controls without SCA or sickle cell trait were enrolled across 4 institutions. Subjects were excluded for 1) inability to perform exercise, 2) receiving chronic transfusions, 3) history of exercise-related arrhythmia/syncope, 4) physician-diagnosed asthma, 5) history of EIB, or 6) history of cardiac disease precluding exercise testing. Participants performed maximal CPET on a cycle ergometer using a standardized Godfrey protocol. At least 2 weeks later, participants performed a controlled intensity interval test (CIIT) consisting of 8 bouts of constant workload cycling randomized to either 50% (moderate intensity) or 70% (vigorous intensity) of peak workload achieved during CPET. Exercise bouts were 2 min each with 1-min rest intervals between bouts. Spirometry was performed pre/post (5, 10, 15, and 30 min) CPET and CIIT. EIB was defined as a drop in FEV1 ≥10% from baseline to the lowest FEV1 recorded. Bivariable comparisons were performed, and logistic regression models analyzed the association between EIB and covariables (age, sex, SCA status, max workload, exercise intensity (CIIT only), baseline FEV1 % predicted, FEV1/FVC % predicted) using IBM ® SPSS ® Statistics.
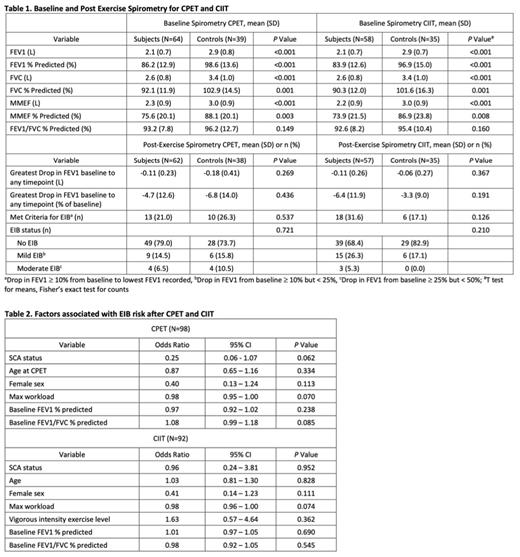
Results Available data from 64 subjects (48% F, 73% on hydroxyurea, mean 13.8±2.8 years) and 40 controls (45% F, mean 15.4±2.9 years) were analyzed. Baseline Hb (8.5±1.2 vs. 13±1.4 g/dL, p < 0.001) and maximal workload achieved during CPET (85±26 vs. 135±45 watts, p < 0.001) were lower in subjects vs. controls. The percentage of participants who completed CIIT did not differ between subjects and controls, and no respiratory-related adverse events, including need for bronchodilator therapy, occurred. At baseline, FEV1 and FEV1 % predicted and other parameters (FVC and FVC % predicted, MMEF and MMEF % predicted) were significantly lower in subjects vs. controls (Table 1). However, there was no between-group difference in baseline FEV1/FVC % predicted. Post-exercise, the percentage of participants who met criteria for EIB did not differ between subjects and controls after CPET (21 vs. 26%, p = 0.537) or CIIT (32 vs. 17%, p = 0.126). There was also no significant between-group difference in the greatest drop in FEV1 from baseline to any post-exercise timepoint (mean value or as % of baseline) after CPET or CIIT. In logistic regression models, SCA status, age, sex, maximal workload during CPET, exercise intensity (CIIT only), baseline FEV1 % predicted and FEV1/FVC % predicted were not associated with EIB after CPET or CIIT (Table 2).
Conclusion Compared to controls, children and young adults with SCA and no history of asthma or EIB had lower baseline values on all spirometry parameters except FEV1/FVC % predicted. EIB was not more common in subjects with SCA vs. controls after maximal CPET or a submaximal exercise challenge of longer duration at moderate or vigorous intensity. No independent factors were associated with development of EIB after acute exercise. Despite concerns that undiagnosed lower airways disease in children with SCA is common, our results suggest the risk of EIB in SCA is low and underscore the safety of moderate to vigorous exercise toward developing evidence-based exercise guidelines in non-asthmatic children and young adults with SCA.
Disclosures
Cohen:Forma Therapeutics: Consultancy, Other: member of a one-time advisory board meeting about clinical trial endpoints ; Sanofi: Other: Member of an independent data safety monitoring board. Hsu:Hilton Publishing, Emmaus, Fresnius: Consultancy; Asklepion Pharmaceuticals: Research Funding; Aruvant: Other: Data Safety Board Member . Tang:NovoNordisk: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Liem:Vertex: Research Funding; NIH/NCATS: Research Funding; NIH/NHLBI: Research Funding; Bluebird Bio: Research Funding; Editas: Research Funding; Global Blood Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal