Sonrotoclax is a potent and selective BCL2 inhibitor that is also effective in venetoclax-resistant BCL2 mutants both in vitro and in vivo.
The crystal structure of BCL2 G101V:sonrotoclax elucidates the molecular basis of overcoming G101V–induced venetoclax resistance.
Visual Abstract
Venetoclax, the first-generation inhibitor of the apoptosis regulator B-cell lymphoma 2 (BCL2), disrupts the interaction between BCL2 and proapoptotic proteins, promoting the apoptosis in malignant cells. Venetoclax is the mainstay of therapy for relapsed chronic lymphocytic leukemia and is under investigation in multiple clinical trials for the treatment of various cancers. Although venetoclax treatment can result in high rates of durable remission, relapse has been widely observed, indicating the emergence of drug resistance. The G101V mutation in BCL2 is frequently observed in patients who relapsed treated with venetoclax and sufficient to confer resistance to venetoclax by interfering with compound binding. Therefore, the development of next-generation BCL2 inhibitors to overcome drug resistance is urgently needed. In this study, we discovered that sonrotoclax, a potent and selective BCL2 inhibitor, demonstrates stronger cytotoxic activity in various hematologic cancer cells and more profound tumor growth inhibition in multiple hematologic tumor models than venetoclax. Notably, sonrotoclax effectively inhibits venetoclax-resistant BCL2 variants, such as G101V. The crystal structures of wild-type BCL2/BCL2 G101V in complex with sonrotoclax revealed that sonrotoclax adopts a novel binding mode within the P2 pocket of BCL2 and could explain why sonrotoclax maintains stronger potency than venetoclax against the G101V mutant. In summary, sonrotoclax emerges as a potential second-generation BCL2 inhibitor for the treatment of hematologic malignancies with the potential to overcome BCL2 mutation–induced venetoclax resistance. Sonrotoclax is currently under investigation in multiple clinical trials.
Introduction
Evasion of apoptosis is one of the hallmarks of tumorigenesis.1,2 BCL2, the first identified intrinsic apoptosis regulator, plays a dominant role in preventing apoptosis in multiple malignant lymphoid cell types.3-5 BCL2 acts by sequestering its proapoptotic substrates, such as BCL2–associated X protein (BAX) and BCL2 antagonist/killer 1 (BAK), and suppressing their function of mediating mitochondrial damage.6-10 BCL2-mediated blockade of the apoptotic signaling pathway is beneficial for the survival of cancer cells. Therefore, disrupting the association between BCL2 and proapoptotic proteins by small-molecule inhibitors to promote apoptosis has long been an attractive strategy for cancer therapy.11-13
Venetoclax, the first BH3 mimetic drug and a selective BCL2 inhibitor, was initially approved for treating relapsed or refractory chronic lymphocytic leukemia (CLL) with del17p in 2016.14-16 Despite some patients achieving remission, relapse has been observed during venetoclax treatment, which also extended to other cancer types in which venetoclax has demonstrated clinical activity.17-23 In a previous study, amplicon sequencing was performed to analyze paired samples from patients who relapsed at treatment initiation and at the disease progression stage, and the result revealed a novel BCL2 G101V mutation present in ∼50% (7/15) of patients at the posttreatment progression stage but not at treatment initiation; and no cases bearing this mutation were detected among 96 patients with venetoclax treatment-naïve CLL, suggesting that G101V is an acquired mutation arising during continuous venetoclax treatment. Moreover, venetoclax exhibits an ∼180-fold decrease in binding affinity to the G101V mutant,24 thus restricting its ability to displace proapoptotic proteins from BCL2. Moreover, RS4;11 and KMS-12PE leukemia cell lines overexpressing BCL2 G101V had reduced sensitivity to venetoclax compared with cells expressing wild-type (WT) BCL2,24,25 confirming that this mutation is closely related to drug resistance. In addition to G101V, other mutations in BCL2, such as D103Y, A113G, R129L, and V156D, have been observed in some patients with disease progression on venetoclax,26-29 however, whether these mutations contribute to venetoclax resistance remains unclear.
To date, venetoclax is the only drug that has been approved for BCL2-targeted therapy, but the emergence of drug resistance related to BCL2 mutations poses a potential treatment hurdle. Consequently, there is an urgent need for BCL2 inhibitors capable of overcoming the mutation–driven drug resistance. To our knowledge, no inhibitors have been reported to be effective against venetoclax-resistant BCL2 mutations. Herein, we discovered that sonrotoclax, a potent and selective BCL2 inhibitor, inhibits both WT BCL2 and several BCL2 mutants. Sonrotoclax demonstrated potent antitumor activity in WT BCL2-, BCL2 G101V-, and BCL2 D103Y-bearing xenograft models. The crystal structure of BCL2 G101V in complex with sonrotoclax elucidated the molecular mechanism by which the compound overcomes G101V mutation–induced drug resistance.
Methods
Protein purification
Human BCL2 (residues 6-207 with amino acids 35-91 replaced with amino acids 33-48 of BCL-xL) was expressed and purified as reported previously.30-32 All mutants were generated by site-directed mutagenesis using a fast mutagenesis system (TransGen Biotech, Beijing, China) and purified with a method similar to that used for WT BCL2.
SPR
Surface plasmon resonance (SPR) experiments were performed on a Biacore 8K system (Cytiva, Marlborough, MA) at room temperature. The binding kinetics of sonrotoclax and venetoclax were measured in HBS-N (HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] buffered saline-NaCl) buffer containing 10 mM HEPES (pH 7.4), 250 mM NaCl, 50 μM EDTA, 0.1% Tween 20, and 1% DMSO (dimethyl sulfoxide). In brief, His-tagged BCL2 or BCL2 variants, including G101V, D103Y, V156D, A113G, and R129L, were immobilized on a nitrilotriacetic acid sensor chip (Cytiva). Venetoclax or sonrotoclax was then flowed over the chip with 240-second injections and 800-second dissociations at a flow rate of 50 μL per minute. The values of the equilibrium binding constant KD were determined through best fit in a 1:1 binding kinetic model.
Cell viability assay
Cell viability was assayed using CellTiter-Glo reagent (Promega, Madison, WI). Cells were treated with stepwise increasing concentrations of the compounds for 2 days. An equal volume of CellTiter-Glo reagent was added to the cell culture medium in each well, and the solution was mixed on an orbital shaker for 2 minutes to allow for cell lysis. To generate a stabilized luminescence signal for quantifying the amount of ATP (adenosine triphosphate) and determining the number of metabolically active cells, the samples were incubated for 10 minutes. The luminescence signal was measured on a PHERAstar FSX reader (BMG Labtech, Ortenburg, Germany). The IC50 (half maximal inhibitory concentration) values of the compounds were determined by fitting the cell viability inhibition at different compound concentrations using the four-parameter logistic model in GraphPad Prism (San Diego, CA).
Xenograft model
Female NCG (NOD CRISPR Prkdc II2rγ) mice were purchased from GemPharmatech Co, Ltd (Nanjing, China). Animals were inoculated with tumor cells in the right flanks and randomized based on tumor volume or transplantation sequence and body weight. After randomization, mice were treated with 10 mL/kg venetoclax or sonrotoclax once daily (QD) via oral gavage, with the dosing regimens and schedules described in the text. Sonrotoclax and venetoclax were formulated for oral administration in 60% (volume/volume [vol/vol]) Phosal 50 PG (propylene glycol), 30% (vol/vol) PEG (polyethylene glycol)-400, and 10% (vol/vol) ethyl alcohol. Tumor volume and body weight were measured twice weekly, and tumor volume was calculated using the following formula: (length × width)2/2. Throughout the study, mice were monitored daily for clinical signs of toxicity. Differences in tumor volume between the sonrotoclax- and venetoclax-treated groups were analyzed using the Welch analysis of variance with the Tamhane T2 test for multiple comparisons; P value <.05 was considered statistically significant. All experiments were performed based on protocols approved by the Animal Care and Use Committee of BeiGene according to the guidelines of the Chinese Association for Laboratory Animal Sciences.
For detailed description of the methods, please refer to the supplemental Materials, available on the Blood website.
Results
Sonrotoclax potently and selectively targets BCL2
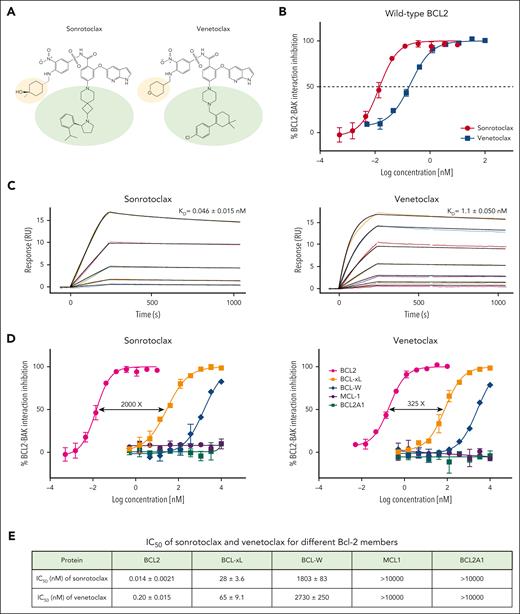
A novel series of BCL2 inhibitors containing 7-azaspiro[3.5]nonane were discovered through scaffold screening and structure-based drug design. Subsequent optimization for potency, selectivity, and drug metabolism and pharmacokinetic properties led to the discovery of sonrotoclax (Figure 1A). To assess its ability to disrupt the interaction between BH3 proteins and BCL2, a parallel investigation of sonrotoclax and venetoclax using cell-free competitive binding assays was conducted. Sonrotoclax inhibited the BCL2-BAK interaction with an IC50 of 0.014 nM, exhibiting a 14-fold increase in potency compared with venetoclax (Figure 1B). Similarly, the SPR experiments revealed that the KD of sonrotoclax to BCL2 was 0.046 nM, indicating a 24-fold improvement in binding affinity over venetoclax (Figure 1C). Sonrotoclax also demonstrated better selectivity than venetoclax for other Bcl-2 family members, particularly BCL-xL, which is related to platelet toxicity and dose-limiting thrombocytopenia.33,34 Sonrotoclax showed a 2000-fold selectivity toward BCL-xL, superior to that of venetoclax (325-fold; Figure 1D-E).
Sonrotoclax is a second-generation BCL2 inhibitor with superior potency and selectivity. (A) Chemical structures of sonrotoclax and venetoclax. The differences are highlighted with light yellow and green ellipses. (B) Cell-free competitive binding assays were performed to measure the disruption of the interaction between the BCL2:BAK-derived peptide by sonrotoclax and venetoclax. (C) SPR binding curves of sonrotoclax and venetoclax. The lines in different colors are the actual curves for the serial concentrations of sonrotoclax (0.625-10 nM) and venetoclax (3.125-200 nM), whereas the corresponding black lines are the fitted curves using the 1:1 binding model. KD values are presented as the mean values ± standard deviations (SDs) for 4 independent experiments. (D) Inhibition of BCL-xL, BCL-W, MCL-1, and BCL2A1 was measured with a method similar to that described in (B). (E) Measured IC50 values of sonrotoclax and venetoclax for the inhibition of Bcl-2 family members. The data are presented as the mean values ± SDs of 3 independent experiments.
Sonrotoclax is a second-generation BCL2 inhibitor with superior potency and selectivity. (A) Chemical structures of sonrotoclax and venetoclax. The differences are highlighted with light yellow and green ellipses. (B) Cell-free competitive binding assays were performed to measure the disruption of the interaction between the BCL2:BAK-derived peptide by sonrotoclax and venetoclax. (C) SPR binding curves of sonrotoclax and venetoclax. The lines in different colors are the actual curves for the serial concentrations of sonrotoclax (0.625-10 nM) and venetoclax (3.125-200 nM), whereas the corresponding black lines are the fitted curves using the 1:1 binding model. KD values are presented as the mean values ± standard deviations (SDs) for 4 independent experiments. (D) Inhibition of BCL-xL, BCL-W, MCL-1, and BCL2A1 was measured with a method similar to that described in (B). (E) Measured IC50 values of sonrotoclax and venetoclax for the inhibition of Bcl-2 family members. The data are presented as the mean values ± SDs of 3 independent experiments.
The cytotoxic activity of sonrotoclax and venetoclax on human platelets was investigated alongside navitoclax, a dual inhibitor of BCL2 and BCL-xL. Navitoclax exhibited a strong platelet-killing effect with an IC50 of 6.4 nM, whereas sonrotoclax and venetoclax showed a limited effect on human platelets (supplemental Figure 1A). Furthermore, sonrotoclax exhibited selectivity comparable with or even slightly higher than that of venetoclax, as indicated by the ratios of IC50 values for both compounds against platelet and RS4;11 cell line (680-fold vs 291-fold) (Figure 2A; supplemental Figure 1B). In addition, both sonrotoclax and venetoclax displayed limited inhibition (<50%) of the viability of CD34+ hematopoietic stem and progenitor cells at concentrations of <1 μM (supplemental Figure 1C).
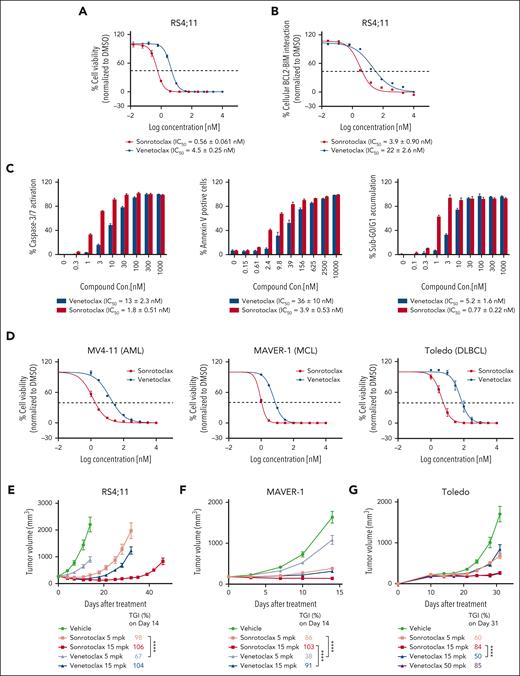
Sonrotoclax is more efficacious than venetoclax in both cancer cells and xenograft mouse models. (A) Cell viability inhibition was measured using a CellTiter-Glo luminescence assay. (B) Disruption of the BCL2:BIM complex was detected by a cell-based competitive Meso Scale Discovery (MSD) assay. (C) The activation of caspase-3 and caspase-7 (caspase-3/7) was measured in RS4;11 cells with a Caspase-Glo kit. Annexin V+ cells were quantified by FITC annexin V staining and FACS analysis in RS4;11 cells. The accumulation of sub-G0/G1 in RS4;11 cells was assessed by PI staining and FACS analysis. The data are presented as the mean values ± SDs for 3 independent experiments, with representative plots shown in the figures. EC50, half maximal effective concentration. (D) MV4-11 (AML), MAVER-1 (MCL), and Toledo (DLBCL) cells were treated with serial dilutions of sonrotoclax or venetoclax for 2 days, and cell viability inhibition was measured by a CellTiter-Glo luminescence assay. IC50 values are presented as the mean values ± SDs for 3 independent experiments. (E-G) The in vivo antitumor activity of sonrotoclax and venetoclax was evaluated in human RS4;11 (E), MAVER-1 (F), and Toledo (G) xenograft models. Mice were treated with sonrotoclax or venetoclax once daily at the indicated doses by oral gavage. The tumor volumes are presented as the mean values ± standard error of the mean (SEMs) of 8 (E) or 10 (F-G) mice in each group; ∗∗∗∗P < .0001. EC50, half maximal effective concentration, FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; mpk, milligrams per kilogram; PI, propidium iodide.
Sonrotoclax is more efficacious than venetoclax in both cancer cells and xenograft mouse models. (A) Cell viability inhibition was measured using a CellTiter-Glo luminescence assay. (B) Disruption of the BCL2:BIM complex was detected by a cell-based competitive Meso Scale Discovery (MSD) assay. (C) The activation of caspase-3 and caspase-7 (caspase-3/7) was measured in RS4;11 cells with a Caspase-Glo kit. Annexin V+ cells were quantified by FITC annexin V staining and FACS analysis in RS4;11 cells. The accumulation of sub-G0/G1 in RS4;11 cells was assessed by PI staining and FACS analysis. The data are presented as the mean values ± SDs for 3 independent experiments, with representative plots shown in the figures. EC50, half maximal effective concentration. (D) MV4-11 (AML), MAVER-1 (MCL), and Toledo (DLBCL) cells were treated with serial dilutions of sonrotoclax or venetoclax for 2 days, and cell viability inhibition was measured by a CellTiter-Glo luminescence assay. IC50 values are presented as the mean values ± SDs for 3 independent experiments. (E-G) The in vivo antitumor activity of sonrotoclax and venetoclax was evaluated in human RS4;11 (E), MAVER-1 (F), and Toledo (G) xenograft models. Mice were treated with sonrotoclax or venetoclax once daily at the indicated doses by oral gavage. The tumor volumes are presented as the mean values ± standard error of the mean (SEMs) of 8 (E) or 10 (F-G) mice in each group; ∗∗∗∗P < .0001. EC50, half maximal effective concentration, FACS, fluorescence-activated cell sorting; FITC, fluorescein isothiocyanate; mpk, milligrams per kilogram; PI, propidium iodide.
Sonrotoclax exhibits superior potency compared with venetoclax in different hematologic cancer cells and xenograft models
To compare the cellular potency of sonrotoclax and venetoclax, their ability to disrupt the BCL2:BIM complex in RS4;11 cells was investigated.35 The IC50 of sonrotoclax was 3.9 nM, which is ∼5 times more potent than venetoclax (Figure 2B). The cell viability assay also demonstrated a potency difference of eightfold between the 2 compounds (Figure 2A). Sonrotoclax induced hallmarks of apoptosis, including caspase activation, externalization of phosphatidylserine, and accumulation of sub-G0/G1 DNA, with sixfold to 10-fold greater effectiveness than venetoclax (Figure 2C). In addition, the cell killing ability of both compounds was investigated in a panel of lymphoma and myeloid leukemia cell lines. Sonrotoclax exhibited more potent cytotoxic activity than venetoclax across multiple cell lines, such as MV4-11 (acute myeloid leukemia [AML]), MAVER-1 (mantle cell lymphoma [MCL]), and Toledo (diffuse large B-cell lymphoma [DLBCL]) (Figure 2D; supplemental Table 1). However, the cell lines with inherent venetoclax resistance were insusceptible to sonrotoclax (supplemental Table 1). In the mechanistic study using KMS-12-PE cells sensitive to BCL2 inhibition, the absence of the downstream mediators of apoptosis, BAK and BAX,36,37 abolished cell killing by both sonrotoclax and venetoclax (supplemental Figure 2), consistent with a previous study.38 This finding suggests that both sonrotoclax and venetoclax kill cells by targeting the BCL2-mediated antiapoptotic pathway.
The in vivo pharmacodynamics and pharmacokinetics of sonrotoclax were evaluated in RS4;11 xenograft-bearing mice. After a single-dose treatment, sonrotoclax induced stronger caspase-3 cleavage in tumors than venetoclax, indicating a more potent induction of apoptosis by sonrotoclax in vivo. This effect can be attributed to the higher potency of sonrotoclax, although a lower concentration of sonrotoclax than venetoclax in tumor was detected (supplemental Figure 3A). In the efficacy study, daily oral administration of sonrotoclax at a low dose of 5 mg/kg produced robust antitumor activity, resulting in a tumor growth inhibition (TGI) rate of 98% on day 14 after treatment, which was significantly higher than that of venetoclax at the same dose (67%; P < .0001). Although at a higher dose of 15 mg/kg, venetoclax initially caused rapid tumor shrinkage, similar to a previous study,39 the tumor relapsed after 21 days of treatment. In contrast, at the same dose, sonrotoclax not only significantly inhibited tumor growth but also delayed tumor relapse to day 39 (Figure 2E). Sonrotoclax also demonstrated superior antitumor activity to venetoclax in xenograft models derived from MAVER-1 (MCL) and Toledo (DLBCL) cells (Figure 2F-G). These results indicate that sonrotoclax has more potent antitumor activity than venetoclax in preclinical models of multiple hematologic cancers. Furthermore, the combination of sonrotoclax and zanubrutinib, a second-generation Bruton tyrosine kinase (BTK) inhibitor,40,41 showed significantly stronger antitumor activity than either single agent in the JeKo-1 xenograft model (supplemental Figure 4), indicating a potential utility of sonrotoclax in combination therapy.
The crystal structure of BCL2 in complex with sonrotoclax reveals a different binding mode in the P2 pocket
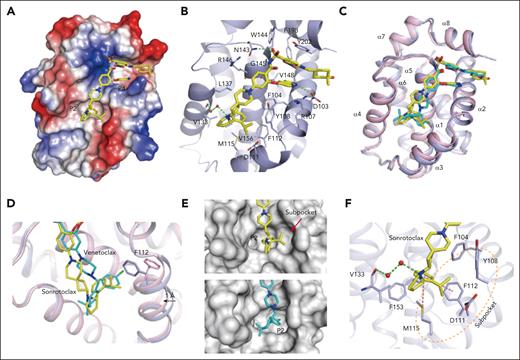
To gain insights into the molecular basis of the interaction between BCL2 and sonrotoclax, the complex structure was determined at a high resolution of 1.80 Å. Sonrotoclax unambiguously bound in the hydrophobic groove of BCL2 (Figure 3A; supplemental Figure 5A) and was stabilized through hydrophobic interactions and hydrogen bonds with surrounding residues (Figure 3B). Superposition of the structures of BCL2 in complex with sonrotoclax and venetoclax revealed a different binding mode of sonrotoclax within the P2 pocket. The (S)-2-(2-isopropylphenyl)pyrrolidine and azaspiro linker of sonrotoclax shifted toward the α4 helix relative to venetoclax, resulting in a longer distance between the isopropylphenyl group of sonrotoclax and the residues of P2 pocket compared with the chlorophenyl group of venetoclax (Figure 3C).42 In the BCL2:venetoclax crystal structure, the chlorophenyl group inserted deeply into the P2 pocket, resulting in a rotamer change of F112 and a movement of the α3 helix by ∼1 Å, through which F112 was relocated to accommodate the interaction. Nonetheless, the isopropylphenyl group of sonrotoclax bound at the entrance of the P2 pocket and formed a beneficial edge-to-face π-π interaction with the side chain of F112, contributing to the stronger binding of sonrotoclax (Figure 3D). The isopropyl substitution induced the formation of a subpocket composed of Y108, F112, D111, and M115 above the P2 site (Figure 3E), providing additional hydrophobic interactions. Moreover, the benzene ring of the sonrotoclax isopropylphenyl group was involved in a sulfur-π interaction with the sulfur atom of M115; pyrrolidine formed a water-bridged hydrogen bond with the main-chain carbonyl of V133, which was absent in the crystal structure of BCL2 in complex with venetoclax (Figure 3F). These interactions collectively contributed to the increased binding affinity of sonrotoclax compared with venetoclax.
Molecular mechanism of the interaction between BCL2 and sonrotoclax. (A) Overview of the structure of BCL2 in complex with sonrotoclax. The protein is shown as an electrostatic surface, with the compound shown in yellow. (B) Binding details of sonrotoclax. Water molecules are shown as red spheres. Hydrogen bonds are represented by green dashed lines. (C) Superimposition of the structures of BCL2 in complex with sonrotoclax (yellow stick) and venetoclax (cyan stick, protein shown as pink ribbon, PDB ID: 6O0K). (D) Zoomed in view of the superimposed structures shown in panel C. The F112 residues underneath the P2 pocket are shown as purple and pink sticks. (E) Comparison of the occupation of the P2 pocket by venetoclax (cyan stick) and sonrotoclax. The subpocket is indicated by a red arrow. (F) Zoomed in view of the binding details of the (S)-2-(2-isopropylphenyl)pyrrolidine moiety. The subpocket is encircled by a light orange dashed line, and the sulfonyl-π and π-π interactions are indicated by purple dashed lines.
Molecular mechanism of the interaction between BCL2 and sonrotoclax. (A) Overview of the structure of BCL2 in complex with sonrotoclax. The protein is shown as an electrostatic surface, with the compound shown in yellow. (B) Binding details of sonrotoclax. Water molecules are shown as red spheres. Hydrogen bonds are represented by green dashed lines. (C) Superimposition of the structures of BCL2 in complex with sonrotoclax (yellow stick) and venetoclax (cyan stick, protein shown as pink ribbon, PDB ID: 6O0K). (D) Zoomed in view of the superimposed structures shown in panel C. The F112 residues underneath the P2 pocket are shown as purple and pink sticks. (E) Comparison of the occupation of the P2 pocket by venetoclax (cyan stick) and sonrotoclax. The subpocket is indicated by a red arrow. (F) Zoomed in view of the binding details of the (S)-2-(2-isopropylphenyl)pyrrolidine moiety. The subpocket is encircled by a light orange dashed line, and the sulfonyl-π and π-π interactions are indicated by purple dashed lines.
Sonrotoclax overcomes BCL2 G101V mutation–related drug resistance
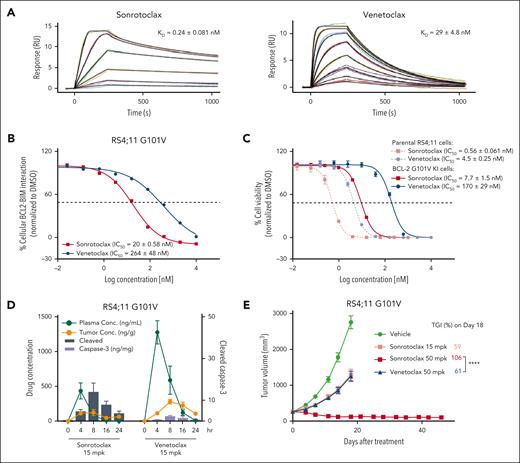
The insufficient binding affinity of venetoclax to BCL2 mutants can result in drug resistance.24 The SPR results demonstrated that the binding affinity of venetoclax to the G101V mutant dropped to 29 nM, a 26-fold decrease compared with that of WT BCL2 (1.1 nM). However, sonrotoclax retained strong binding affinity to this mutant, with a KD of 0.24 nM (Figure 4A). To evaluate the cellular potency of sonrotoclax toward the BCL2 G101V mutant, RS4;11 cells with knockin (KI) of the BCL2 G101V were generated. The incorporation of the G101V mutation was confirmed at the DNA and RNA levels (supplemental Figure 6A). BCL2 expression level in KI cells was comparable with that of parental cells (supplemental Figure 6B). In RS4;11 G101V KI cells, sonrotoclax exhibited 13-fold higher potency than venetoclax in disrupting the BCL2 G101V:BIM complex (Figure 4B). Consistent with this finding, RS4:11 G101V KI cells were 22-fold more sensitive to sonrotoclax than to venetoclax. Notably, the IC50 of sonrotoclax in the G101V KI cells was 7.7 nM, which was nearly equivalent to that of venetoclax in the parental RS4;11 cells (Figure 4C). Collectively, these results indicate that sonrotoclax is effective against the BCL2 G101V mutant in cancer cells.
Sonrotoclax is effective against the venetoclax-resistant BCL2 G101V mutant. (A) Binding affinities of sonrotoclax (left panel) and venetoclax (right panel) to the BCL2 G101V mutant measured by SPR. The curves in different colors represent the serial concentrations of sonrotoclax (0.625-20 nM) and venetoclax (6.25-800 nM). KD values are presented as the mean values ± SDs for 4 independent experiments. (B) The disruption of the cellular BCL2:BIM complex in BCL2 G101V KI RS4;11 cells by sonrotoclax and venetoclax were detected using the MSD assay. (C) Inhibition of the cell viability in the parental RS4;11 and G101V KI cells was evaluated by a CellTiter-Glo luminescence assay. IC50 values are presented as the mean values ± SDs for 3 independent experiments, with representative curves shown in panels B and C. (D-E) Assessment of in vivo PD/PK and efficacy of sonrotoclax and venetoclax in RS4;11 G101V xenograft models. Mice were treated with sonrotoclax or venetoclax at the indicated doses. Single-dose treatment was used to evaluate PD, and daily dosing was used for the efficacy study; all treatments were administered by oral gavage. For evaluation of PD/PK, plasma and tumor tissues were collected at the specified time points to measure drug concentrations and tumor cleaved caspase-3 (Asp175) levels by ELISA. For the efficacy study, the tumor volume was measured twice weekly, and the TGI was calculated on day 18 after dosing. The data are presented as the mean values ± SEMs of 3 mice at each time point (D) and 8 mice in each group (E); ∗∗∗∗P < .0001. ELISA, enzyme linked immunosorbent assay; PD, pharmacodynamics; PK, pharmacokinetics.
Sonrotoclax is effective against the venetoclax-resistant BCL2 G101V mutant. (A) Binding affinities of sonrotoclax (left panel) and venetoclax (right panel) to the BCL2 G101V mutant measured by SPR. The curves in different colors represent the serial concentrations of sonrotoclax (0.625-20 nM) and venetoclax (6.25-800 nM). KD values are presented as the mean values ± SDs for 4 independent experiments. (B) The disruption of the cellular BCL2:BIM complex in BCL2 G101V KI RS4;11 cells by sonrotoclax and venetoclax were detected using the MSD assay. (C) Inhibition of the cell viability in the parental RS4;11 and G101V KI cells was evaluated by a CellTiter-Glo luminescence assay. IC50 values are presented as the mean values ± SDs for 3 independent experiments, with representative curves shown in panels B and C. (D-E) Assessment of in vivo PD/PK and efficacy of sonrotoclax and venetoclax in RS4;11 G101V xenograft models. Mice were treated with sonrotoclax or venetoclax at the indicated doses. Single-dose treatment was used to evaluate PD, and daily dosing was used for the efficacy study; all treatments were administered by oral gavage. For evaluation of PD/PK, plasma and tumor tissues were collected at the specified time points to measure drug concentrations and tumor cleaved caspase-3 (Asp175) levels by ELISA. For the efficacy study, the tumor volume was measured twice weekly, and the TGI was calculated on day 18 after dosing. The data are presented as the mean values ± SEMs of 3 mice at each time point (D) and 8 mice in each group (E); ∗∗∗∗P < .0001. ELISA, enzyme linked immunosorbent assay; PD, pharmacodynamics; PK, pharmacokinetics.
In vivo pharmacodynamics/pharmacokinetics of the compounds were evaluated in RS4;11 BCL2 G101V xenografts. Sonrotoclax at 15 mg/kg induced caspase-3 cleavage, whereas venetoclax had little effect at the same dose (Figure 4D). Administration of sonrotoclax at 50 mg/kg resulted in rapid tumor regression in all 8 mice (TGI of 106% on day 18), which was sustained until day 46 (Figure 4E), without causing significant body weight loss (supplemental Figure 3B). In contrast, venetoclax at 50 mg/kg exhibited inadequate efficacy, with a TGI of only 61% (Figure 4E). The results suggest the therapeutic potential of sonrotoclax for the treatment of BCL2 G101V mutation-harboring tumors.
The crystal structure of BCL2 G101V in complex with sonrotoclax elucidates the molecular mechanism of overcoming G101V-induced drug resistance
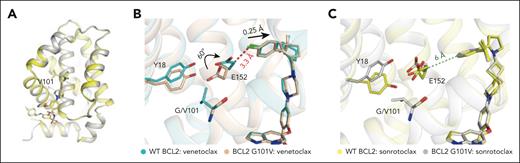
The crystal structure of BCL2 G101V mutant in complex with sonrotoclax was determined at 1.90 Å. The overall structure was well superimposed with the BCL2 WT:sonrotoclax structure, with a root mean square deviation of 0.28 Å for 131 Cα atoms, and no significant difference of the binding pose of sonrotoclax was observed between the both structures (Figure 5A). A previous study showed that in the BCL2 G101V:venetoclax structure, the bulky side chain of residue V101 induced conformational changes in several nearby residues, particularly E152. The side chain of E152 rotated by a 60° angle toward the P2 pocket, leading to repositioning of venetoclax and hindering compound binding through a knockon effect (Figure 5B).42
Crystal structure of the BCL2 G101V mutant in complex with sonrotoclax. (A) Comparison of the structures of WT BCL2 (yellow) and the G101V mutant (gray) in complex with sonrotoclax. (B-C) Conformational changes of residues around G/V101 in the crystal structures of WT BCL2 and G101V in complex with venetoclax (B) and sonrotoclax (C). The complex structures of BCL2:venetoclax (PDB ID: 6O0K) and BCL2 G101V:venetoclax (PDB ID: 6O0L) are shown in cyan and light orange, respectively. The minimum distances between E152 of the BCL2 G101V mutant and the compounds are indicated by green and red dashed lines.
Crystal structure of the BCL2 G101V mutant in complex with sonrotoclax. (A) Comparison of the structures of WT BCL2 (yellow) and the G101V mutant (gray) in complex with sonrotoclax. (B-C) Conformational changes of residues around G/V101 in the crystal structures of WT BCL2 and G101V in complex with venetoclax (B) and sonrotoclax (C). The complex structures of BCL2:venetoclax (PDB ID: 6O0K) and BCL2 G101V:venetoclax (PDB ID: 6O0L) are shown in cyan and light orange, respectively. The minimum distances between E152 of the BCL2 G101V mutant and the compounds are indicated by green and red dashed lines.
Although the side chain of E152 adopted dual conformations in the BCL2 WT:sonrotoclax structure, both rotamers were distant from the compound, and the shortest distance from Cβ of E152 to the isopropylphenyl group of sonrotoclax was ∼6 Å (supplemental Figure 5B). Despite the mutated valine residue pushing E152 toward the compound, the shortest distance remained ∼6 Å (Figure 5C), preventing a steric clash between sonrotoclax and E152. Thus, the knockon effect of BCL2 G101V mutation had a lesser effect on the binding of sonrotoclax. The structural observation explains how sonrotoclax maintains high potency against the BCL2 G101V mutant.
Sonrotoclax potently inhibits other BCL2 variants
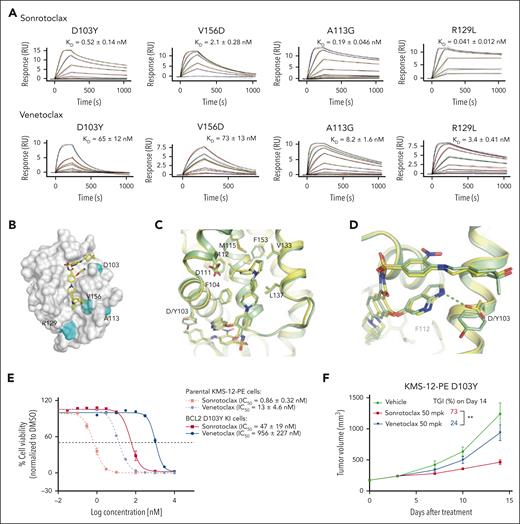
In addition to G101V, other mutations have been detected in patients who relapsed treated with venetoclax,28 but how these mutants affect compound binding remains unclear. To assess the binding affinities of sonrotoclax and venetoclax to these mutants, SPR experiments were performed. Venetoclax displayed reduced binding affinities to the BCL2 mutants D103Y and V156D compared with WT BCL2 (a decrease of ∼60-fold). However, the binding affinity of venetoclax to the R129L and A113G mutants only decreased modestly (by threefold to eightfold). Similarly, sonrotoclax retained its KD to the R129L mutant, and only showed a fourfold lower binding affinity to the A113G mutant. Meanwhile, the binding affinities of sonrotoclax to the D103Y and V156D mutants decreased to varying degrees (Figure 1C; Figure 6A). However, sonrotoclax displayed 125-fold higher affinity than venetoclax to the D103Y mutant and 35 to 83-fold higher affinity to the A113G, V156D, and R129L mutants (Figure 6A).
Sonrotoclax remains effective against other BCL2 mutants. (A) SPR measurements of the binding affinities of sonrotoclax and venetoclax to different BCL2 variants. The curves in different colors represent different concentrations of sonrotoclax (0.156-20 nM for D103Y, 0.313-20 nM for V156D, 0.078-20 nM for A113G, and 1.25-20 nM for R129L) and venetoclax (0.781-200 nM for D103Y, 1.56-100 nM for V156D, 0.781-400 nM for A113G, and 0.781-200 nM for R129L). KD values are presented as the mean values ± SDs for 4 independent experiments. (B) Mutations are indicated on the BCL2 protein surface (gray). The mutated residues are highlighted in cyan. Sonrotoclax is represented by the yellow stick. (C) Superimposition of the structures of WT BCL2 (yellow) and the D103Y mutant (pale green) in complex with sonrotoclax. (D) Zoomed in view of D103 or Y103 involved in the binding of sonrotoclax or venetoclax. The hydrogen bonds are indicated by the cyan and yellow dashed lines. (E) Inhibition of the viability of parental and BCL2 D103Y KI KMS-12-PE cells was estimated by the CellTiter-Glo luminescence assay. IC50 values are presented as the mean values ± SDs for 3 independent experiments, with representative curves shown in the figure. (F) Efficacy evaluation of sonrotoclax and venetoclax in the KMS-12-PE D103Y xenograft model. The tumor volumes are presented as the mean values ± SEMs of 8 animals in each group; ∗∗P < .01.
Sonrotoclax remains effective against other BCL2 mutants. (A) SPR measurements of the binding affinities of sonrotoclax and venetoclax to different BCL2 variants. The curves in different colors represent different concentrations of sonrotoclax (0.156-20 nM for D103Y, 0.313-20 nM for V156D, 0.078-20 nM for A113G, and 1.25-20 nM for R129L) and venetoclax (0.781-200 nM for D103Y, 1.56-100 nM for V156D, 0.781-400 nM for A113G, and 0.781-200 nM for R129L). KD values are presented as the mean values ± SDs for 4 independent experiments. (B) Mutations are indicated on the BCL2 protein surface (gray). The mutated residues are highlighted in cyan. Sonrotoclax is represented by the yellow stick. (C) Superimposition of the structures of WT BCL2 (yellow) and the D103Y mutant (pale green) in complex with sonrotoclax. (D) Zoomed in view of D103 or Y103 involved in the binding of sonrotoclax or venetoclax. The hydrogen bonds are indicated by the cyan and yellow dashed lines. (E) Inhibition of the viability of parental and BCL2 D103Y KI KMS-12-PE cells was estimated by the CellTiter-Glo luminescence assay. IC50 values are presented as the mean values ± SDs for 3 independent experiments, with representative curves shown in the figure. (F) Efficacy evaluation of sonrotoclax and venetoclax in the KMS-12-PE D103Y xenograft model. The tumor volumes are presented as the mean values ± SEMs of 8 animals in each group; ∗∗P < .01.
Mapping of BCL2 mutations revealed that the R129L and A113G mutations are located far from the hydrophobic groove and are likely passenger mutations.43 In comparison, the V156D and D103Y mutations are located in the P2 and P4 pockets, respectively, and involved in the direct interaction with venetoclax and sonrotoclax (Figure 6B). Thus, both BCL2 mutations will interfere with the protein-compound association, possibly explaining why sonrotoclax and venetoclax maintained their binding affinities to the R129L and A113G but not to the V156D and D103Y mutants. Specifically, the residue D103 formed a hydrogen bond with the azaindole substitution of venetoclax and sonrotoclax (Figures 6B and 3B),34 which is critical for compound binding.
To better understand the molecular mechanism underlying the interaction between sonrotoclax and the BCL2 D103Y mutant, we solved the complex structure at 2.25 Å. The overall structure was well superimposed with the BCL2 WT:sonrotoclax structure, with a root mean square deviation of 0.43 Å for 128 Cα atoms (Figure 6C). The major difference between the 2 structures was that D103 interacted with the azaindole of sonrotoclax through a hydrogen bond, whereas the substitution of aspartic acid with tyrosine disrupted the interaction (Figure 6D). Although the side chain of the tyrosine compensated for the interaction via hydrophobic contact with the azaindole group, this mutation impaired the binding of sonrotoclax and venetoclax. However, the binding affinity of sonrotoclax to the D103Y mutant was still in the subnanomolar range and was ∼130-fold stronger than that of venetoclax (Figures 1C and 6A). Thus, we speculate that the hydrogen bond between D103 and the azaindole group might contribute less to the binding affinity of sonrotoclax than that of venetoclax. In addition, the methyl group of the cyclohexanol moiety of sonrotoclax, which is absent from the tetrahydropyran of venetoclax, also engaged in hydrophobic interaction with the phenol group of Y103, partially rescuing the decreased binding affinity of sonrotoclax to BCL2 D103Y mutant (Figure 6D).
Subsequently, KMS-12-PE D103Y KI cells were generated (supplemental Figure 6A,C) and treated with sonrotoclax and venetoclax. Sonrotoclax showed ∼20-fold more potent inhibition of cell viability than that of venetoclax in D103Y KI cells (Figure 6E), indicating that sonrotoclax remains effective against the D103Y mutant in the cellular context. Consistent with this finding, sonrotoclax at 50 mg/kg showed robust antitumor activity in the KMS-12-PE D103Y xenograft model, whereas venetoclax at the same dose exhibited almost no antitumor effect. The TGIs on day 14 were 73% and 24% for sonrotoclax and venetoclax, respectively (Figure 6F).
Venetoclax resistance can result from mechanisms other than BCL2 mutations.44,45 To investigate the activity of sonrotoclax in other resistance models, venetoclax-resistant RS4;11 cell line was generated.46 The resistant cells, with increased MCL1 protein level rather than BCL2 mutations, were not susceptible to sonrotoclax (supplemental Figure 7A-B). In addition, neither BCL2 mutations nor significant changes of the messenger RNA levels of antiapoptotic proteins was detected in relapsed tumor samples (supplemental Figure 7C-D). However, the downregulation of the proapoptotic protein BAX was observed in sonrotoclax and venetoclax treatment groups (supplemental Figure 7D). It has also been reported that BAX downregulation or acquired mutations in BAX can confer venetoclax resistance.47-49 In summary, these data suggest that venetoclax resistance caused by mechanisms other than BCL2 mutations cannot be overcome by sonrotoclax.
Discussion
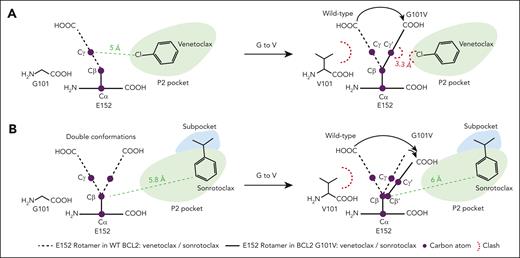
Several studies have demonstrated the frequent occurrence of the BCL2 G101V mutation in patients with relapsed CLL treated with venetoclax. Blombery et al analyzed paired samples obtained before venetoclax treatment and at the progression stage from 15 patients with CLL and identified the G101V mutation in 7 of 15 patients at progression.24 Subsequently, Tausch et al reported the appearance of the G101V mutation in 3 of 4 patients who became refractory to venetoclax.27 In a broader patient cohort, Kuang et al detected the G101V-associated allele in peripheral blood–derived mononuclear cells in 9 of 28 patients.50 The G101V mutation impairs the interaction between venetoclax and BCL2, thereby conferring drug resistance.24 The mutation might be an early indicator of imminent disease progression and lead to failure of venetoclax retreatment. In this study, we discovered sonrotoclax, a second-generation BCL2 inhibitor, with superior potency and selectivity compared with venetoclax. Sonrotoclax exhibited pronounced efficacy in cancer cells and xenograft models bearing the G101V mutation, indicating its potential to overcome venetoclax resistance conferred by this mutation. Regarding the molecular mechanism, the G101V mutation induced a rotamer change in E152, resulting in venetoclax repositioning and a subsequent decrease in the binding affinity of the compound (Figure 7A).42 However, in the BCL2 G101V:sonrotoclax structure, G101V is too distant from sonrotoclax to interfere with its binding, even via E152 (Figure 7B). Therefore, the interaction between sonrotoclax and BCL2 was less affected by the knockon effect of the BCL2 G101V mutation.
Schematic diagram of the knockon effect of the V101 side chain against E152. (A) G101V mutation interferes with BCL2-venetoclax interaction through E152. (B) G101V mutation is too distant from sonrotoclax to impede its binding, even via E152. The legends are shown below the diagrams.
Schematic diagram of the knockon effect of the V101 side chain against E152. (A) G101V mutation interferes with BCL2-venetoclax interaction through E152. (B) G101V mutation is too distant from sonrotoclax to impede its binding, even via E152. The legends are shown below the diagrams.
Although BCL2 G101V is the most commonly reported mutation, other mutations in BCL2 have been detected in patients who relapsed treated with venetoclax.28 Of the BCL2 mutations examined in this study, we found that venetoclax displayed reduced affinity to the D103Y mutant. Cells bearing the D103Y mutation were less sensitive to venetoclax compared with parental cells, suggesting that this mutation conferred venetoclax resistance. Notably, sonrotoclax is still effective against the D103Y mutant in the cellular context and the xenograft model (Figure 6E-F), indicating that sonrotoclax could overcome the D103Y mutation–driven drug resistance. Moreover, sonrotoclax demonstrated stronger binding affinities to other BCL2 mutants detected in patients to date (Figure 6A), which might decrease the chance that these mutations emerge under continuous exposure to sonrotoclax. Nonetheless, future studies will be needed to determine whether BCL2 mutations occur in patients treated with sonrotoclax.
Although our data showed that sonrotoclax can overcome BCL2 mutation–induced venetoclax resistance in cancer cells and xenograft models, resistance caused by other mechanisms, such as mutation, downregulation of proapoptotic proteins, or upregulation of non-BCL2 antiapoptotic proteins,48,51-53 cannot be overcome by sonrotoclax. Combination therapies, such as cotargeting BCL2 and MCL1, might hold promise for circumventing venetoclax resistance. However, the relevant role of MCL1 in cardiac function may compromise the safety of MCL1 inhibitors.54 BTK inhibition decreases MCL1 and BCL-xL levels,55-57 sensitizing CLL cells to BCL2 inhibition. Moreover, anti-CD20 monoclonal antibodies can downregulate BCL-xL and resensitize resistant cells to BCL2 inhibition.58,59 Venetoclax resistance caused by mechanisms not related to BCL2 mutations may be potentially overcome through combinations of BCL2 inhibitors with BTK inhibitors and anti-CD20 monoclonal antibodies.60,61
In conclusion, sonrotoclax is a promising next-generation BCL2 inhibitor that is effective against both WT BCL2 and several mutants. It has the potential for treating treatment-naïve patients as well as those resistant to venetoclax due to BCL2 mutations. Furthermore, combining sonrotoclax with other anticancer drugs that use different mechanism of action presents a therapeutic landscape for hematologic malignancies. Sonrotoclax as a monotherapy or in combination with other anticancer agents for the treatment of various cancers is under clinical investigation (NCT04277637, NCT04771130, NCT04883957, NCT05471843, and NCT05479994).
Acknowledgments
The authors thank the staff of beamlines BL02U1/BL18U1/BL19U1 at the Shanghai Synchrotron Radiation Facility for their assistance during data collection. The authors also thank Enago and Springer Nature for language review.
Authorship
Contribution: Y.F. performed the protein purification and crystallization of WT BCL2 and BCL2 mutants in complex with sonrotoclax; J.L., M.X., and Y.H. determined the crystal structures; Ying Guo and Xiaoxin Liu performed the TR-FRET and SPR assays; Xuefei Yang, Z.M., H. Xing, Y.Z., Z.C., and S.C. performed the cellular BCL2-BIM binding, cell viability, and other cellular assays in cultured cell lines; W.Z., N.W., L.L., and H.W. generated the venetoclax-resistant cell lines and performed the cell viability assays; J.W. analyzed the sequencing data of relapsed tumor samples; X. Luan and Q.W. analyzed and interpreted the cellular data; S.L., S.Y., Y.M., S.Z., A.X., and Xiaolong Yang performed the efficacy and PK/PD studies; N.H. supervised the in vivo studies; H. Xue synthesized the compounds; Y.L., X.S., X. Yuan, and Yunhang Guo designed and supervised the overall study; Y.L., J.L., S.L., Q.W., M.X., Xiaoxin Liu, and H.S. wrote the manuscript with input from all authors; Xuesong Liu and Z.W. reviewed and commented on the manuscript; and all authors have approved the final version of the manuscript.
Conflict-of-interest disclosure: All authors are current or previous employees and shareholders of BeiGene (Beijing) Co, Ltd. The design of, conduct of, and financial support for this research were provided by BeiGene (Beijing) Co, Ltd. BeiGene (Beijing) Co, Ltd, participated in data interpretation and the review and approval of the manuscript.
Correspondence: Ye Liu, Department of Molecular Science, BeiGene (Beijing) Co, Ltd, No 30 Science Park Rd, Zhong-Guan-Cun Life Science Park, Changping District, Beijing 102206, People’s Republic of China; email: ye.liu@beigene.com.
References
Author notes
J.L. and S.L. contributed equally to this study.
Coordinates and structure factors have been deposited in the Protein Data Bank (accession numbers 8HOG, 8HOH, and 8HOI). All other relevant data supporting the key findings are available within the article and its supplementary files.
Data are available upon reasonable request from the corresponding author, Ye Liu (ye.liu@beigene.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal