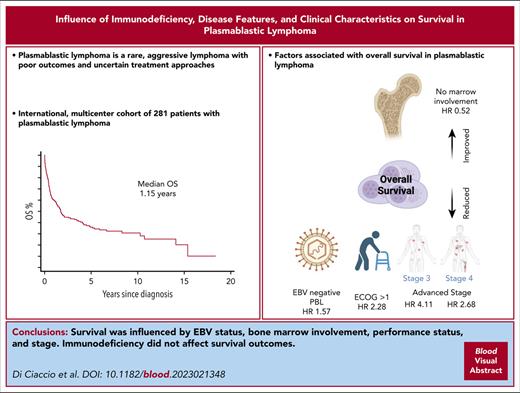
PBL is a rare non-Hodgkin lymphoma with a poor prognosis and uncertainty regarding optimal treatment approaches.
Advanced stage, bone marrow involvement, ECOG > 1, and EBV–negative lymphoma are associated with inferior OS.
Visual Abstract
Plasmablastic lymphoma (PBL) is a rare and aggressive non-Hodgkin lymphoma associated with immunodeficiency, characterized by uncertain treatment approaches and an unfavorable prognosis. We conducted a multicenter, international, retrospective cohort study, aiming to characterize the clinical features, risk factors, and outcomes of patients with PBL. Data were collected from 22 institutions across 4 countries regarding patients diagnosed with PBL between 1 January 1999 and 31 December 2020. Survival risk factors were analyzed using both univariate and multivariate regression models. Overall survival (OS) was calculated using Kaplan-Meier statistics. First-line treatment regimens were stratified into standard- and higher-intensity regimens, and based on whether they incorporated a proteasome inhibitor (PI). A total of 281 patients (median age, 55 years) were included. Immunodeficiency of any kind was identified in 144 patients (51%), and 99 patients (35%) had HIV-positive results. The 5-year OS for the entire cohort was 36% (95% confidence interval, 30%-42%). In multivariate analysis, inferior OS was associated with Epstein-Barr virus–negative lymphoma, poor performance status, advanced stage, and bone marrow involvement. In an independent univariate analysis, the international prognostic index was associated with OS outcomes. Neither immunosuppression nor HIV infection, specifically, influenced OS. Among patients treated with curative intent (n = 234), the overall response rate was 72%. Neither the intensity of the treatment regimen nor the inclusion of PIs in first-line therapy was associated with OS. In this large retrospective study of patients with PBL, we identified novel risk factors for survival. PBL remains a challenging disease with poor long-term outcomes.
Introduction
Plasmablastic lymphoma (PBL) is a rare aggressive subtype of B-cell non-Hodgkin lymphoma, frequently associated with HIV infection; however, it is also seen in patients with other forms of immunosuppression as well as patients with apparent immunocompetence.1,2
PBL classically presents as an extranodal disease involving the oral cavity, but it may also arise at various other nodal and extranodal sites. Patients frequently present with aggressive disease and advanced stage.3 The cell of origin is the terminal plasmablast with downregulation of B-cell transcription factors, such as PAX5. Expression of plasma cell markers CD38 and CD138 is common, and only a small minority will retain weak expression of B-cell markers, such as CD20.3-5 It is postulated that MYC dysregulation disrupts the physiological maturation of plasmablasts to terminally differentiated plasma cells.6 Infection of malignant cells with Epstein-Barr virus (EBV) has been reported, particularly in HIV-associated cases, with a frequency of >70% in that setting.1,7
Given the rarity of PBL, there is no standard therapeutic approach. The prognosis of PBL is generally poor, with a reported 3 to 5-year overall survival (OS) rate of ∼40%.8-10 Prospective evidence is lacking, with the existing data mostly limited to case reports and modest retrospective studies. Risk factors for survival and how they may be used to inform treatment decisions are typically not well established in the literature, with conflicting results.2,7,9,11
We aimed to perform an international, multicenter, retrospective analysis to evaluate the clinical features, treatment, and outcomes of patients with PBL, with a view to identifying prognostic factors that may be used to inform treatment decisions and improve patient outcomes.
Methods
We retrospectively identified PBL cases from 22 contributing sites in Australia, the United Kingdom, Canada, and Singapore. Ethics approval for the study was granted by the Human Research Ethics Committee at St. Vincent’s Hospital, Sydney, Australia (2020/ETH00484) and the institutional review boards in contributing jurisdictions.
Patients were included if they had a tissue biopsy confirming the diagnosis of PBL between 1 January 1999 and 31 December 2020 and were aged at least 18 years. Although a centralized pathology review was not performed, all contributing sites were major academic centers with experienced hematopathologists and robust internal multidisciplinary review processes for lymphoproliferative disorders. Patients with human herpesvirus 8-associated lymphoma, anaplastic lymphoma kinase (ALK)-associated lymphoma, plasmacytoma, or high-grade B-cell lymphoma, not otherwise specified, were excluded according to the World Health Organization diagnostic criteria for PBL.4
Descriptive statistics were compared using the χ2 test for categorical variables and Wilcoxon rank-sum test for continuous variables. OS was estimated using standard time-to-event analyses, including Kaplan-Meier statistics, from the date of diagnosis to death or the last follow-up. To account for loss to follow-up (LTFU) and heterogeneity across contributing centers, factors associated with OS were analyzed using Fine and Gray competing risk regression, adjusted for contributing centers. OS was determined from the date of diagnosis to the date of death. Patients known to be alive were censored on the date of their last follow-up. LTFU was analyzed as a competing risk, and such patients were censored on their date of LTFU. Patients with <1 day of follow-up were excluded.
Risk factors included in the survival analysis were age, biological sex, HIV status, MYC rearrangement status, EBV status, immunophenotype (CD19, CD20, and CD30; by flow cytometry or immunohistochemistry), lactate dehydrogenase, advanced stage (III-IV), Eastern Cooperative Oncology Group (ECOG) performance status, central nervous system (CNS) involvement, bone marrow (BM) involvement, standard- vs higher-intensity first-line treatment, incorporation of rituximab, incorporation of a proteasome inhibitor (PI), autologous stem cell transplant (ASCT) in first line, international prognostic index (IPI) score, and primary tumor location. Primary tumor location was categorized into 2 groups: oropharyngeal, nasopharyngeal, orbital, jaw, and upper neck and all other sites. All variables were analyzed as time-fixed covariates. A sensitivity analysis was performed to determine the effects of immunosuppression status on survival among patients with known HIV status.
The regression models were fitted using a backward stepwise selection process. Risk factors found to be significant in the univariate model at P < .10 were included in the multivariate model. Risk factors with P < .05 in the multivariate model were considered statistically significant.
EBV biopsy status was identified by either EBV-encoded small RNA, EBV nuclear antigen 2, or latency membrane protein-1. MYC rearrangement was determined by fluorescence in situ hybridization, regardless of the partner gene. Immunosuppression-related PBL was defined as a case associated with either HIV, solid organ, or allogeneic stem cell transplant, primary immunodeficiency, immunosuppressive medication (including ≥20 mg per day of prednisone or equivalent), or an underlying indolent lymphoproliferative disease. BM involvement was detected using either positron emission tomography (PET) or direct BM biopsy.
Stage and response were assessed based on the 2014 Lugano consensus criteria.12 Curative intent treatment was defined as a first-line treatment containing either an anthracycline and/or consolidated with high-dose chemotherapy and ASCT. Curative intent regimens were further classified as either standard intensity (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP] or CHOP-like) or higher intensity. A detailed classification of the treatment regimens is provided in the supplemental Appendix, available on the Blood website (supplemental Table 1). All patients were included in the survival analysis irrespective of the treatment received.
Data management and statistical analyses were performed using statistical analysis system (version 9.4; SAS Institute Inc, Cary, NC), Stata software version 16.1 (Stata Corp, College Station, TX), and GraphPad Prism software version 9.2.0 (GraphPad Inc, San Diego, CA).
Data for 42 patients contributed by BC Cancer, University of British Columbia, Vancouver, British Columbia, Canada, have been published separately and previously, following the primary data analysis of this study.16
Results
Patient characteristics
A total of 281 patients from 22 sites were included in the analysis. The patient characteristics are described in Table 1. The median age at diagnosis was 55 years (interquartile range [IQR], 44-69 years), and the majority of patients were male (199 patients [71%]). Seventy-nine patients (28%) had primary tumor sites classically associated with PBL in the head or neck, as defined in “Methods.” Most patients were diagnosed with advanced-stage disease, and 143 (51%) with stage IV disease, specifically.
Patient characteristics, including comparison based on immunosuppression status
| . | Total patients . | Immunosuppression related . | Not immunosuppression related . | P value . |
|---|---|---|---|---|
| n = 281 (%) . | n = 144 (%) . | n = 115 (%) . | ||
| Age at diagnosis (y) | Median = 55 (IQR, 44-69) | Median = 48 (IQR, 42-57) | Median = 64.9 (IQR, 52-73) | <.001 |
| ≤40 | 47 (17) | 28 (19) | 17 (15) | <.001 |
| 41-65 | 151 (54) | 99 (69) | 42 (37) | |
| >65 | 83 (30) | 17 (12) | 56 (49) | |
| Sex | ||||
| Male | 199 (71) | 104 (72) | 81 (70) | .752 |
| Female | 82 (29) | 40 (28) | 34 (30) | |
| Year of diagnosis | .112 | |||
| ≤2010 | 83 (30) | 51 (35) | 27 (23) | |
| 2011-2015 | 99 (35) | 45 (31) | 44 (38) | |
| 2016-2020 | 99 (35) | 48 (33) | 44 (38) | |
| Stage | ||||
| I | 68 (24) | 31 (22) | 33 (29) | .012 |
| II | 45 (16) | 19 (13) | 24 (21) | |
| III | 17 (6) | 11 (8) | 5 (4) | |
| IV | 143 (51) | 82 (57) | 47 (41) | |
| Not reported | 8 (3) | 1 (1) | 6 (5) | |
| HIV status | ||||
| Positive | 99 (35) | 99 (69) | 0 (0) | <.001 |
| Negative | 160 (57) | 45 (31) | 115 (100) | |
| Not tested/unknown | 22 (8) | 0 (0) | 0 (0) | |
| MYC rearrangement | ||||
| Yes | 52 (19) | 31 (22) | 19 (17) | <.001 |
| No | 41 (15) | 9 (6) | 29 (25) | |
| Not tested/unknown | 188 (67) | 104 (72) | 67 (58) | |
| EBV status | ||||
| Positive | 160 (57) | 103 (72) | 50 (43) | <.001 |
| Negative | 73 (26) | 26 (18) | 38 (33) | |
| Not tested/unknown | 48 (17) | 15 (10) | 27 (23) | |
| CD20 | ||||
| Positive | 26 (9) | 13 (9) | 11 (10) | <.001 |
| Weak | 11 (4) | 5 (3) | 5 (4) | |
| Negative | 217 (77) | 102 (71) | 98 (85) | |
| Not tested/unknown | 27 (10) | 24 (17) | 1 (1) | |
| CD30 | ||||
| Positive | 44 (16) | 21 (15) | 18 (16) | .846 |
| Negative | 116 (41) | 59 (41) | 50 (43) | |
| Not tested/unknown | 121 (43) | 64 (44) | 47 (41) | |
| BM involvement | ||||
| Yes | 51 (18) | 29 (20) | 17 (15) | .472 |
| No | 169 (60) | 89 (62) | 73 (63) | |
| Not tested/unknown | 61 (22) | 26 (18) | 25 (22) | |
| CNS involvement | ||||
| Yes | 13 (5) | 9 (6) | 3 (3) | .005 |
| No | 183 (65) | 105 (73) | 68 (59) | |
| Not tested/unknown | 85 (30) | 30 (21) | 44 (38) | |
| Location of primary tumor | ||||
| Oropharynx/nasopharynx/orbit | 79 (28) | 38 (26) | 40 (35) | .101 |
| Other site | 181 (64) | 93 (65) | 71 (62) | |
| Not reported/unknown | 21 (7) | 13 (9) | 4 (3) | |
| LDH level | ||||
| Elevated | 155 (55) | 82 (57) | 62 (54) | .458 |
| Not elevated | 87 (31) | 42 (29) | 41 (36) | |
| Not tested/unknown | 39 (14) | 20 (14) | 12 (10) | |
| ECOG performance status | ||||
| ≤1 | 182 (65) | 89 (62) | 87 (76) | .046 |
| >1 | 84 (30) | 48 (33) | 26 (23) | |
| Not reported | 15 (5) | 7 (5) | 2 (2) | |
| Frontline treatment | ||||
| High intensity | 75 (27) | 38 (26) | 32 (28) | .837 |
| Standard intensity | 159 (57) | 86 (60) | 68 (59) | |
| Other | 43 (15) | 19 (13) | 15 (13) | |
| Not reported | 4 (1) | 1 (1) | 0 (0) | |
| Rituximab | ||||
| Yes | 45 (16) | 23 (16) | 22 (19) | .505 |
| No | 236 (84) | 121 (84) | 93 (81) | |
| PI-containing regimen | ||||
| Yes | 33 (12) | 14 (10) | 19 (17) | .066 |
| No | 205 (73) | 90 (63) | 76 (66) | |
| Not reported | 43 (15) | 40 (28) | 20 (17) | |
| ASCT in first line | ||||
| Yes | 13 (5) | 3 (2) | 10 (9) | .016 |
| No | 268 (95) | 141 (98) | 105 (91) | |
| IPI | ||||
| Low risk | 76 (27) | 40 (28) | 34 (30) | .406 |
| Low-intermediate risk | 49 (17) | 21 (15) | 25 (22) | |
| High-intermediate risk | 68 (24) | 36 (25) | 29 (25) | |
| High risk | 36 (13) | 20 (14) | 10 (9) | |
| Not reported | 52 (19) | 27 (19) | 17 (15) |
| . | Total patients . | Immunosuppression related . | Not immunosuppression related . | P value . |
|---|---|---|---|---|
| n = 281 (%) . | n = 144 (%) . | n = 115 (%) . | ||
| Age at diagnosis (y) | Median = 55 (IQR, 44-69) | Median = 48 (IQR, 42-57) | Median = 64.9 (IQR, 52-73) | <.001 |
| ≤40 | 47 (17) | 28 (19) | 17 (15) | <.001 |
| 41-65 | 151 (54) | 99 (69) | 42 (37) | |
| >65 | 83 (30) | 17 (12) | 56 (49) | |
| Sex | ||||
| Male | 199 (71) | 104 (72) | 81 (70) | .752 |
| Female | 82 (29) | 40 (28) | 34 (30) | |
| Year of diagnosis | .112 | |||
| ≤2010 | 83 (30) | 51 (35) | 27 (23) | |
| 2011-2015 | 99 (35) | 45 (31) | 44 (38) | |
| 2016-2020 | 99 (35) | 48 (33) | 44 (38) | |
| Stage | ||||
| I | 68 (24) | 31 (22) | 33 (29) | .012 |
| II | 45 (16) | 19 (13) | 24 (21) | |
| III | 17 (6) | 11 (8) | 5 (4) | |
| IV | 143 (51) | 82 (57) | 47 (41) | |
| Not reported | 8 (3) | 1 (1) | 6 (5) | |
| HIV status | ||||
| Positive | 99 (35) | 99 (69) | 0 (0) | <.001 |
| Negative | 160 (57) | 45 (31) | 115 (100) | |
| Not tested/unknown | 22 (8) | 0 (0) | 0 (0) | |
| MYC rearrangement | ||||
| Yes | 52 (19) | 31 (22) | 19 (17) | <.001 |
| No | 41 (15) | 9 (6) | 29 (25) | |
| Not tested/unknown | 188 (67) | 104 (72) | 67 (58) | |
| EBV status | ||||
| Positive | 160 (57) | 103 (72) | 50 (43) | <.001 |
| Negative | 73 (26) | 26 (18) | 38 (33) | |
| Not tested/unknown | 48 (17) | 15 (10) | 27 (23) | |
| CD20 | ||||
| Positive | 26 (9) | 13 (9) | 11 (10) | <.001 |
| Weak | 11 (4) | 5 (3) | 5 (4) | |
| Negative | 217 (77) | 102 (71) | 98 (85) | |
| Not tested/unknown | 27 (10) | 24 (17) | 1 (1) | |
| CD30 | ||||
| Positive | 44 (16) | 21 (15) | 18 (16) | .846 |
| Negative | 116 (41) | 59 (41) | 50 (43) | |
| Not tested/unknown | 121 (43) | 64 (44) | 47 (41) | |
| BM involvement | ||||
| Yes | 51 (18) | 29 (20) | 17 (15) | .472 |
| No | 169 (60) | 89 (62) | 73 (63) | |
| Not tested/unknown | 61 (22) | 26 (18) | 25 (22) | |
| CNS involvement | ||||
| Yes | 13 (5) | 9 (6) | 3 (3) | .005 |
| No | 183 (65) | 105 (73) | 68 (59) | |
| Not tested/unknown | 85 (30) | 30 (21) | 44 (38) | |
| Location of primary tumor | ||||
| Oropharynx/nasopharynx/orbit | 79 (28) | 38 (26) | 40 (35) | .101 |
| Other site | 181 (64) | 93 (65) | 71 (62) | |
| Not reported/unknown | 21 (7) | 13 (9) | 4 (3) | |
| LDH level | ||||
| Elevated | 155 (55) | 82 (57) | 62 (54) | .458 |
| Not elevated | 87 (31) | 42 (29) | 41 (36) | |
| Not tested/unknown | 39 (14) | 20 (14) | 12 (10) | |
| ECOG performance status | ||||
| ≤1 | 182 (65) | 89 (62) | 87 (76) | .046 |
| >1 | 84 (30) | 48 (33) | 26 (23) | |
| Not reported | 15 (5) | 7 (5) | 2 (2) | |
| Frontline treatment | ||||
| High intensity | 75 (27) | 38 (26) | 32 (28) | .837 |
| Standard intensity | 159 (57) | 86 (60) | 68 (59) | |
| Other | 43 (15) | 19 (13) | 15 (13) | |
| Not reported | 4 (1) | 1 (1) | 0 (0) | |
| Rituximab | ||||
| Yes | 45 (16) | 23 (16) | 22 (19) | .505 |
| No | 236 (84) | 121 (84) | 93 (81) | |
| PI-containing regimen | ||||
| Yes | 33 (12) | 14 (10) | 19 (17) | .066 |
| No | 205 (73) | 90 (63) | 76 (66) | |
| Not reported | 43 (15) | 40 (28) | 20 (17) | |
| ASCT in first line | ||||
| Yes | 13 (5) | 3 (2) | 10 (9) | .016 |
| No | 268 (95) | 141 (98) | 105 (91) | |
| IPI | ||||
| Low risk | 76 (27) | 40 (28) | 34 (30) | .406 |
| Low-intermediate risk | 49 (17) | 21 (15) | 25 (22) | |
| High-intermediate risk | 68 (24) | 36 (25) | 29 (25) | |
| High risk | 36 (13) | 20 (14) | 10 (9) | |
| Not reported | 52 (19) | 27 (19) | 17 (15) |
Bold values are statistically significant.
Regarding the staging modality, 134 were staged by PET and 92 by computerized tomography (other/missing data, n = 55). BM involvement was demonstrated in 51 of the 220 patients who underwent BM biopsy. CNS involvement was relatively uncommon (13 patients [5%]), with a mixture of cases involving the leptomeninges and the cortical parenchyma. CNS-IPI13 risk factors for patients with CNS involvement are described in supplemental Table 2.
Analyzing the 259 patients with known HIV status, we compared patient characteristics according to their immunodeficiency status (Table 1). Immunodeficiency, including but not limited to HIV, was a factor in 144 (56%) patients. Patients with immunodeficiency were younger (median, 48 vs 65 years; P < .001) and were more likely to have EBV-positive PBL (72% vs 43%; P < .001) and MYC rearrangements (22% vs 17%; P < .001).
A minority of patients (n = 99; 35%) had HIV-positive results. Of the patients with HIV-positive results, 63 (64%) were on antiretroviral therapy at the time of PBL diagnosis. Another 25 patients (25% of the total patients with HIV) commenced antiretroviral therapy after PBL diagnosis. The median CD4 count and HIV viral load at PBL diagnosis were 208 × 106/L (IQR, 65-334) and 252 copies per mL (IQR, 40-68 500) respectively.
Survival
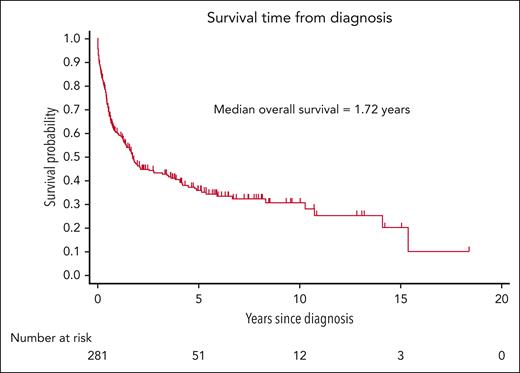
The median duration of follow-up was 1.15 years (IQR, 0.41-3.67 years). There were 167 deaths (59%), and 33 patients (12%) were lost to follow-up. The median OS was 1.72 years (95% confidence interval [CI], 1.3-2.8), with an estimated 5-year OS rate of 36% (95% CI, 30-42; Figure 1). The relatively short follow-up period was predominantly because of early deaths rather than censoring events, with a median time to mortality of 0.52 years (IQR, 0.17-1.61) among the 167 patients who died.
The most common cause of death was progressive PBL (117 patients [70%]), followed by infection (19 patients [11%]), subsequent primary malignancy (10 patients [6%]), and noninfectious treatment-related causes (5 patients [3%]). Nine patients died of other miscellaneous causes, and 8 did not have available cause-of-death data.
Risk factors for survival
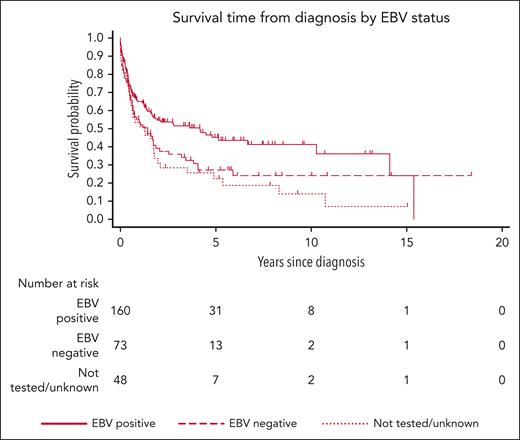
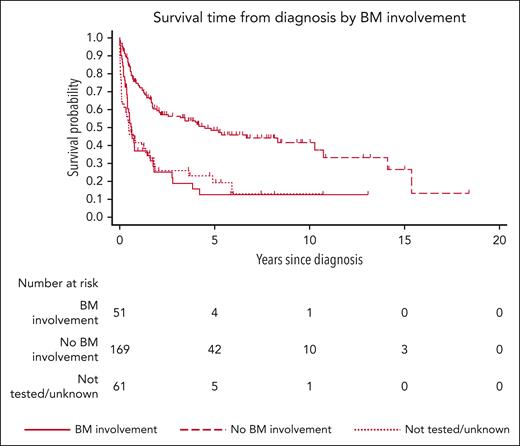
The results of the risk factor analysis are presented in Table 2. In univariate analysis, factors associated with increased mortality risk were older age, female sex, negative EBV status, advanced stage, BM involvement, CNS involvement, primary tumor located outside the head/neck, and ECOG performance status > 1. On multivariate analysis, however, only negative EBV status (subhazard ratio [SHR], 1.57; 95% CI, 1.03-2.39; P = .037), advanced stage compared with stage I (stage III SHR, 4.11; 95% CI, 2.04-8.28; P < .001; stage IV SHR, 2.68; 95% CI, 1.62-4.44; P < .001, respectively), ECOG performance status > 1 (SHR, 2.28; 95% CI, 1.50-3.48; P < .001; Figures 2 and 3; supplemental Figure 1), and other front line treatment, compared with high-intensity treatment (SHR, 6.52; 95% CI, 3.68-11.55; P < .001), were associated with higher mortality. Absence of BM involvement (SHR, 0.52; 95% CI, 0.32-0.83; P = .006) was associated with a superior OS. There was no interaction between BM and disease stage (P = .163). The proportional hazards assumption was satisfied for all variables in the regression model.
Risk factors associated with mortality from diagnosis of PBL
| . | No. of patients . | Follow-up (y) . | No. of deaths . | Mortality rate (per 100 py) . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | |||||
| Total | 281 | 707.3 | 167 | 23.6 | ||||||
| Age at diagnosis (y) | .001 | |||||||||
| ≤40 | 47 | 134.5 | 26 | 19.3 | 1 | |||||
| 41-65 | 151 | 415.5 | 83 | 20.0 | 0.86 | (0.54-1.38) | .537 | |||
| >65 | 83 | 157.3 | 58 | 36.9 | 1.78 | (1.07-2.98) | .028 | |||
| Sex | ||||||||||
| Male | 199 | 548.1 | 109 | 19.9 | 1 | |||||
| Female | 82 | 159.2 | 58 | 36.4 | 1.45 | (1.05-2.01) | .024 | |||
| Year of diagnosis | .832 | |||||||||
| ≤2010 | 83 | 334.1 | 59 | 17.7 | 1 | |||||
| 2011-2015 | 99 | 261.7 | 60 | 22.9 | 1.04 | (0.71-1.53) | .822 | |||
| 2016-2020 | 99 | 111.5 | 48 | 43.0 | 1.14 | (0.74-1.76) | .547 | |||
| Stage | <.001 | <.001 | ||||||||
| I | 68 | 243.2 | 22 | 9.0 | 1 | 1 | ||||
| II | 45 | 153.5 | 23 | 15.0 | 1.44 | (0.81-2.55) | .217 | 1.45 | (0.79-2.68) | .233 |
| III | 17 | 54.2 | 13 | 24.0 | 2.87 | (1.45-5.69) | .003 | 4.11 | (2.04-8.28) | <.001 |
| IV | 143 | 242.3 | 102 | 42.1 | 3.17 | (1.97-5.11) | <.001 | 2.68 | (1.62-4.44) | <.001 |
| Not reported | 8 | 14.2 | 7 | 49.4 | ||||||
| HIV status | .470 | |||||||||
| Positive | 99 | 303.8 | 54 | 17.8 | 1 | |||||
| Negative | 160 | 386.0 | 92 | 23.8 | 1.16 | (0.77-1.75) | .470 | |||
| Not tested/unknown | 22 | 17.5 | 21 | 120.3 | ||||||
| MYC rearrangement | .869 | |||||||||
| Yes | 52 | 119.7 | 28 | 23.4 | 1 | |||||
| No | 41 | 109.6 | 23 | 21.0 | 0.95 | (0.51-1.76) | .869 | |||
| Not tested/unknown | 188 | 478.0 | 116 | 24.3 | ||||||
| EBV status | .033 | |||||||||
| Positive | 160 | 428.0 | 80 | 18.7 | 1 | 1 | ||||
| Negative | 73 | 174.7 | 50 | 28.6 | 1.52 | (1.03-2.22) | .033 | 1.57 | (1.03-2.39) | .037 |
| Not tested/unknown | 48 | 104.6 | 37 | 35.4 | ||||||
| CD20+ | .313 | |||||||||
| Yes | 26 | 60.5 | 17 | 28.1 | 1 | |||||
| Weak | 11 | 20.5 | 8 | 39.1 | 1.64 | (0.74-3.60) | .220 | |||
| No | 217 | 544.2 | 124 | 22.8 | 0.97 | (0.60-1.56) | .889 | |||
| Not tested/unknown | 27 | 82.1 | 18 | 21.9 | ||||||
| CD19+ | .214 | |||||||||
| Yes | 10 | 20.3 | 4 | 19.7 | 1 | |||||
| No | 33 | 67.6 | 20 | 29.6 | 2.09 | (0.65-6.70) | .214 | |||
| Not tested/unknown | 238 | 619.4 | 143 | 23.1 | ||||||
| CD30+ | .601 | |||||||||
| Yes | 44 | 103.4 | 29 | 28.0 | 1 | |||||
| No | 116 | 318.0 | 61 | 19.2 | 0.88 | (0.54-1.42) | .601 | |||
| Not tested/unknown | 121 | 285.9 | 77 | 26.9 | ||||||
| BM involvement | <.001 | |||||||||
| Yes | 51 | 71.7 | 39 | 54.4 | 1 | 1 | ||||
| No | 169 | 553.7 | 83 | 15.0 | 0.42 | (0.28-0.64) | <.001 | 0.52 | (0.32-0.83) | .006 |
| Not tested/unknown | 61 | 82.0 | 45 | 54.9 | ||||||
| CNS involvement | .009 | |||||||||
| Yes | 13 | 13.4 | 11 | 82.3 | 1 | |||||
| No | 183 | 504.9 | 97 | 19.2 | 0.37 | (0.18-0.78) | .009 | |||
| Not tested/unknown | 85 | 189.0 | 59 | 31.2 | ||||||
| Location of primary tumor | <.001 | |||||||||
| Oropharynx/nasopharynx/orbit | 79 | 292.6 | 27 | 9.2 | 1 | |||||
| Other site | 181 | 356.2 | 128 | 35.9 | 2.60 | (1.72-3.94) | <.001 | |||
| Not reported/unknown | 21 | 58.5 | 12 | 20.5 | ||||||
| LDH level | <.001 | |||||||||
| Elevated | 155 | 365.2 | 102 | 27.9 | 1 | |||||
| Not elevated | 87 | 250.9 | 34 | 13.6 | 0.49 | (0.33-0.73) | <.001 | |||
| Not tested/unknown | 39 | 91.2 | 31 | 34.0 | ||||||
| ECOG performance status | <.001 | |||||||||
| ≤1 | 182 | 583.8 | 80 | 13.7 | 1 | 1 | ||||
| >1 | 84 | 101.4 | 74 | 73.0 | 3.79 | (2.58-5.59) | <.001 | 2.28 | (1.50-3.48) | <.001 |
| Not reported | 15 | 22.1 | 13 | 58.9 | ||||||
| Frontline treatment | <.001 | <.001 | ||||||||
| High intensity | 75 | 229.4 | 34 | 14.8 | 1 | 1 | ||||
| Standard intensity | 159 | 433.5 | 90 | 20.8 | 1.06 | (0.71-1.59) | .773 | 1.40 | (0.86-2.28) | .173 |
| Other | 43 | 44.4 | 39 | 87.8 | 4.43 | (2.60-7.56) | <.001 | 6.52 | (3.68-11.55) | <.001 |
| Not reported | 4 | 0.0 | 4 | 14 610.0 | ||||||
| Rituximab | ||||||||||
| Yes | 45 | 111.4 | 27 | 24.2 | 1 | |||||
| No | 236 | 595.9 | 140 | 23.5 | 1.1 | (0.72-1.67) | .669 | |||
| PI-containing regimen | .195 | |||||||||
| Yes | 33 | 62.9 | 12 | 19.1 | 1 | |||||
| No | 205 | 600.5 | 116 | 19.3 | 1.52 | (0.81-2.88) | .195 | |||
| Not reported | 43 | 44.0 | 39 | 88.7 | ||||||
| ASCT in first line | ||||||||||
| Yes | 13 | 59.0 | 5 | 8.5 | 1 | |||||
| No | 268 | 648.3 | 162 | 25.0 | 2.02 | (0.97-4.23) | .061 | |||
| IPI | <.001 | |||||||||
| Low risk | 76 | 272.7 | 19 | 7.0 | 1 | |||||
| Low-intermediate risk | 49 | 153.3 | 27 | 17.6 | 2.34 | (1.35-4.05) | .002 | |||
| High-intermediate risk | 68 | 121.2 | 48 | 39.6 | 3.90 | (2.28-6.69) | <.001 | |||
| High risk | 36 | 40.9 | 32 | 78.1 | 8.08 | (4.43-14.76) | <.001 | |||
| Not reported | 52 | 119.2 | 41 | 34.4 | ||||||
| . | No. of patients . | Follow-up (y) . | No. of deaths . | Mortality rate (per 100 py) . | Univariate . | Multivariate . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SHR . | 95% CI . | P value . | SHR . | 95% CI . | P value . | |||||
| Total | 281 | 707.3 | 167 | 23.6 | ||||||
| Age at diagnosis (y) | .001 | |||||||||
| ≤40 | 47 | 134.5 | 26 | 19.3 | 1 | |||||
| 41-65 | 151 | 415.5 | 83 | 20.0 | 0.86 | (0.54-1.38) | .537 | |||
| >65 | 83 | 157.3 | 58 | 36.9 | 1.78 | (1.07-2.98) | .028 | |||
| Sex | ||||||||||
| Male | 199 | 548.1 | 109 | 19.9 | 1 | |||||
| Female | 82 | 159.2 | 58 | 36.4 | 1.45 | (1.05-2.01) | .024 | |||
| Year of diagnosis | .832 | |||||||||
| ≤2010 | 83 | 334.1 | 59 | 17.7 | 1 | |||||
| 2011-2015 | 99 | 261.7 | 60 | 22.9 | 1.04 | (0.71-1.53) | .822 | |||
| 2016-2020 | 99 | 111.5 | 48 | 43.0 | 1.14 | (0.74-1.76) | .547 | |||
| Stage | <.001 | <.001 | ||||||||
| I | 68 | 243.2 | 22 | 9.0 | 1 | 1 | ||||
| II | 45 | 153.5 | 23 | 15.0 | 1.44 | (0.81-2.55) | .217 | 1.45 | (0.79-2.68) | .233 |
| III | 17 | 54.2 | 13 | 24.0 | 2.87 | (1.45-5.69) | .003 | 4.11 | (2.04-8.28) | <.001 |
| IV | 143 | 242.3 | 102 | 42.1 | 3.17 | (1.97-5.11) | <.001 | 2.68 | (1.62-4.44) | <.001 |
| Not reported | 8 | 14.2 | 7 | 49.4 | ||||||
| HIV status | .470 | |||||||||
| Positive | 99 | 303.8 | 54 | 17.8 | 1 | |||||
| Negative | 160 | 386.0 | 92 | 23.8 | 1.16 | (0.77-1.75) | .470 | |||
| Not tested/unknown | 22 | 17.5 | 21 | 120.3 | ||||||
| MYC rearrangement | .869 | |||||||||
| Yes | 52 | 119.7 | 28 | 23.4 | 1 | |||||
| No | 41 | 109.6 | 23 | 21.0 | 0.95 | (0.51-1.76) | .869 | |||
| Not tested/unknown | 188 | 478.0 | 116 | 24.3 | ||||||
| EBV status | .033 | |||||||||
| Positive | 160 | 428.0 | 80 | 18.7 | 1 | 1 | ||||
| Negative | 73 | 174.7 | 50 | 28.6 | 1.52 | (1.03-2.22) | .033 | 1.57 | (1.03-2.39) | .037 |
| Not tested/unknown | 48 | 104.6 | 37 | 35.4 | ||||||
| CD20+ | .313 | |||||||||
| Yes | 26 | 60.5 | 17 | 28.1 | 1 | |||||
| Weak | 11 | 20.5 | 8 | 39.1 | 1.64 | (0.74-3.60) | .220 | |||
| No | 217 | 544.2 | 124 | 22.8 | 0.97 | (0.60-1.56) | .889 | |||
| Not tested/unknown | 27 | 82.1 | 18 | 21.9 | ||||||
| CD19+ | .214 | |||||||||
| Yes | 10 | 20.3 | 4 | 19.7 | 1 | |||||
| No | 33 | 67.6 | 20 | 29.6 | 2.09 | (0.65-6.70) | .214 | |||
| Not tested/unknown | 238 | 619.4 | 143 | 23.1 | ||||||
| CD30+ | .601 | |||||||||
| Yes | 44 | 103.4 | 29 | 28.0 | 1 | |||||
| No | 116 | 318.0 | 61 | 19.2 | 0.88 | (0.54-1.42) | .601 | |||
| Not tested/unknown | 121 | 285.9 | 77 | 26.9 | ||||||
| BM involvement | <.001 | |||||||||
| Yes | 51 | 71.7 | 39 | 54.4 | 1 | 1 | ||||
| No | 169 | 553.7 | 83 | 15.0 | 0.42 | (0.28-0.64) | <.001 | 0.52 | (0.32-0.83) | .006 |
| Not tested/unknown | 61 | 82.0 | 45 | 54.9 | ||||||
| CNS involvement | .009 | |||||||||
| Yes | 13 | 13.4 | 11 | 82.3 | 1 | |||||
| No | 183 | 504.9 | 97 | 19.2 | 0.37 | (0.18-0.78) | .009 | |||
| Not tested/unknown | 85 | 189.0 | 59 | 31.2 | ||||||
| Location of primary tumor | <.001 | |||||||||
| Oropharynx/nasopharynx/orbit | 79 | 292.6 | 27 | 9.2 | 1 | |||||
| Other site | 181 | 356.2 | 128 | 35.9 | 2.60 | (1.72-3.94) | <.001 | |||
| Not reported/unknown | 21 | 58.5 | 12 | 20.5 | ||||||
| LDH level | <.001 | |||||||||
| Elevated | 155 | 365.2 | 102 | 27.9 | 1 | |||||
| Not elevated | 87 | 250.9 | 34 | 13.6 | 0.49 | (0.33-0.73) | <.001 | |||
| Not tested/unknown | 39 | 91.2 | 31 | 34.0 | ||||||
| ECOG performance status | <.001 | |||||||||
| ≤1 | 182 | 583.8 | 80 | 13.7 | 1 | 1 | ||||
| >1 | 84 | 101.4 | 74 | 73.0 | 3.79 | (2.58-5.59) | <.001 | 2.28 | (1.50-3.48) | <.001 |
| Not reported | 15 | 22.1 | 13 | 58.9 | ||||||
| Frontline treatment | <.001 | <.001 | ||||||||
| High intensity | 75 | 229.4 | 34 | 14.8 | 1 | 1 | ||||
| Standard intensity | 159 | 433.5 | 90 | 20.8 | 1.06 | (0.71-1.59) | .773 | 1.40 | (0.86-2.28) | .173 |
| Other | 43 | 44.4 | 39 | 87.8 | 4.43 | (2.60-7.56) | <.001 | 6.52 | (3.68-11.55) | <.001 |
| Not reported | 4 | 0.0 | 4 | 14 610.0 | ||||||
| Rituximab | ||||||||||
| Yes | 45 | 111.4 | 27 | 24.2 | 1 | |||||
| No | 236 | 595.9 | 140 | 23.5 | 1.1 | (0.72-1.67) | .669 | |||
| PI-containing regimen | .195 | |||||||||
| Yes | 33 | 62.9 | 12 | 19.1 | 1 | |||||
| No | 205 | 600.5 | 116 | 19.3 | 1.52 | (0.81-2.88) | .195 | |||
| Not reported | 43 | 44.0 | 39 | 88.7 | ||||||
| ASCT in first line | ||||||||||
| Yes | 13 | 59.0 | 5 | 8.5 | 1 | |||||
| No | 268 | 648.3 | 162 | 25.0 | 2.02 | (0.97-4.23) | .061 | |||
| IPI | <.001 | |||||||||
| Low risk | 76 | 272.7 | 19 | 7.0 | 1 | |||||
| Low-intermediate risk | 49 | 153.3 | 27 | 17.6 | 2.34 | (1.35-4.05) | .002 | |||
| High-intermediate risk | 68 | 121.2 | 48 | 39.6 | 3.90 | (2.28-6.69) | <.001 | |||
| High risk | 36 | 40.9 | 32 | 78.1 | 8.08 | (4.43-14.76) | <.001 | |||
| Not reported | 52 | 119.2 | 41 | 34.4 | ||||||
Global P values were test for heterogeneity, excluding not reported/unknown values. All analyses were adjusted for site.
Risk factors that were found to be significant in the univariate model at P < .10 were included in the multivariate model. Results of those risk factors found to be significant in the multivariate model are shown.
LDH, lactate dehydrogenase; py, person years.
Values highlighted in bold represent significant covariates in the final multivariate model.
The IPI was associated with survival in the univariate analysis (low-intermediate risk: SHR, 2.34; 95% CI, 1.35-4.05; P = .002; high-intermediate risk: SHR, 3.90; 95% CI, 2.28-6.69; P < .001; and high risk: SHR, 8.08; 95% CI, 4.43-14.76; P < .001, compared with low risk) but was not included in the multivariate analysis because of collinearity with other variables (supplemental Figure 2).
Immunodeficiency was not associated with OS in a sensitivity analysis (SHR, 1.14; 95% CI, 0.78-1.67; P = .490).
Treatment strategies for patients treated with curative intent
First-line treatment
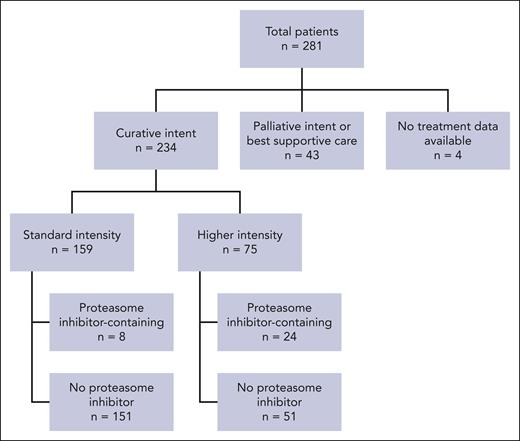
A total of 234 patients received therapy with curative intent, of whom 159 received standard-intensity regimens and 75 received higher-intensity regimens (Figure 4). The most common standard-intensity regimen was CHOP (n = 110). The most common high-intensity regimen was dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (EPOCH; n = 17), followed by EPOCH plus bortezomib (n = 15) (supplemental Table 1). Treatment intensity was not associated with age, IPI, or stage; however, patients treated with a higher-intensity regimen were more likely to have ECOG performance status ≤ 1 (P = .0012).
The overall and complete response (CR) rates were 72% (n = 169) and 59% (n = 137), respectively (assessment modality: PET, n = 116; plain computerized tomography, n = 60; and other/data not available, n = 58). Response data were missing for 9% of the patients (n = 22). There was no effect on either the overall response rate (ORR; P = .21) or OS (P = .773) for patients who received higher-intensity regimens compared with that for those who received standard-intensity regimens (Table 2).
Thirty-two patients (12%) received a chemotherapy regimen that incorporated PI (31 bortezomib and 1 carfilzomib). There was no difference in ORR (P = .51) or OS (P = .195) between patients exposed to a PI as a first-line treatment and those who were not (Table 2).
Twelve patients (5%) underwent consolidation ASCT as part of first-line therapy, 11 in CR and 1 in partial response; the latter case achieved CR after ASCT. Thirty-six patients treated with curative intent received consolidation radiotherapy after initial chemotherapy. Most of these patients had stage I disease (n = 27) and primary oropharyngeal or orbital PBL (n = 28).
CNS involvement and prophylaxis
Among the patients treated with curative intent, 9 (4%) had CNS involvement at the time of diagnosis. Only 1 of these patients was alive at the last follow-up, who had PBL confined to the CNS and was treated with high-dose methotrexate (HDMTX) and high-dose cytarabine.
A total of 27 patients received CNS-directed prophylaxis, comprising HDMTX alone for 7, intrathecal prophylaxis for 19, and both HDMTX and intrathecal for 1 patient. Twenty-five of these patients had extranodal disease sites, and 13 had elevated lactate dehydrogenase level. No CNS relapses were observed in this group; however, CNS relapses among the whole cohort were rare (n = 3; 1%).
Relapsed or progressive disease
Among 169 patients with an initial response to curative intent treatment, 59 relapsed or progressed (35%). Analyzing only patients who achieved CR in after first-line treatment, 38 (28%) relapsed. The median time to first relapse/progression from the date of diagnosis was 8.4 months (IQR, 6.0-14.4 months). The latest relapse occurred ∼5 years after diagnosis.
A wide variety of treatments were used for relapsed/refractory PBL. The ORR to second-line treatment was 44% (n = 19). The median OS after the first relapse or progression of patients initially treated with curative intent was 5.3 months (IQR, 1.9-12.6 months; supplemental Figure 3). Eleven patients received ASCT as part of second-line treatment, with 5 achieving sustained CR.
A total of 5 patients received allogeneic hematopoietic cell transplant (allo-HCT) for relapsed/refractory PBL. Four of these patients died, 3 owing to transplant complications and 1 owing to a second malignancy. One patient who underwent allo-HCT after 1 prior line of treatment was alive at last follow-up, 26 months after allo-HCT, with moderate chronic graft-versus-host disease.
Palliative treatment strategies
A total of 43 patients received palliative chemotherapy or best supportive care (Figure 4). Two patients were managed with a reduction in immunosuppression alone, both of whom achieved CR with this strategy. The first patient was a 37-year-old solid-organ transplant recipient with HIV-negative status and EBV-negative PBL of the skin and who subsequently died of secondary acute myeloid leukemia. The second patient was a 54-year-old allo-HCT recipient with HIV-negative status and stage IE PBL who was alive at last follow-up.
Discussion
We conducted an international, multicenter, retrospective analysis to evaluate the clinical features of a large cohort of patients with PBL and define important clinical risk factors for survival.
The key finding of our study was the identification of several independent risk factors for OS by multivariate analysis. Specifically, advanced stage at presentation, BM involvement, poor performance status, and EBV-negative lymphoma were independently associated with a worse OS. To our knowledge, the adverse effects of both BM involvement and EBV-negative PBL as independent risk factors are novel findings.
Furthermore, we validated the IPI as a useful tool for predicting survival in PBL. A retrospective cohort study of 135 patients with PBL found the IPI to be prognostic when stratified into low- (IPI, 0-2) and high-risk (IPI, 3-5) cohorts.9 Our study has importantly been able to develop this finding further by confirming the prognostic ability of the IPI in PBL in terms of the 4 original risk stratifications of the IPI as independent predictors of survival.14
Although PBL is classically associated with immunodeficient states, particularly HIV infection, 44% of patients in this study were immunocompetent at diagnosis. These patients were older, raising the prospect of contributory age-related immunosenescence; however, this requires further study.15 Patients who were immunocompetent in this study were less likely to have disease associated with EBV infection and MYC rearrangements.
The association between EBV-negative PBL tumors and inferior survival supports the findings of a smaller recent Canadian study, which found a similar association, albeit only on univariate analysis.16 As previously observed,9,11 immunosuppression-related PBL cases in our study were enriched for EBV involvement. There is evidence that EBV-negative PBL demonstrates a distinct molecular signature with greater molecular complexity and mutational load.17 The prognostic impact of various mutational signatures on prognosis is an area of potential future study.
The lack of impact of HIV on survival in this study contrasts with the findings of Tchernonog et al, who found that HIV was a predictor of favorable outcomes.9 However, another series as well as a large national registry study found that HIV status was not associated with OS.7,8 We expanded this analysis to consider all patients with immunosuppression as a whole, not only those with HIV, and similarly, no association with OS was found.
Our study demonstrated a modest 5-year OS rate of 36%, confirming the aggressive nature and relatively poor prognosis of PBL, although several patients had long-term remissions and a likely functional cure. Recent registry-level data from the National Cancer Database and the Surveillance, Epidemiology and End Results Program describe 3-year OS rates of 40% and 54%, respectively,8,18 which is broadly consistent with the findings of our study. Smaller studies have reported a median OS of 6 to 18 months, albeit often with very wide CIs.1,2,7,10,11
Owing to the notable heterogeneity in baseline characteristics and treatment methods, conclusions about treatment efficacy from this study should be interpreted cautiously and viewed as hypothesis-generating only. Within the confines of this important limitation, neither the use of higher-intensity regimens nor the inclusion of PIs in first-line treatment enhanced survival in this study.
The guidelines of the National Comprehensive Cancer Network suggest that CHOP is inadequate for the treatment of PBL; however, within the limitations of retrospective research, no study has yet shown a survival advantage with the use of more intense regimens.7,9,11 The use of plasma cell–directed therapies, such as PIs, has a promising biological basis but is yet to be confirmed in prospective studies.19,20
The potential for successful incorporation of more novel approaches in the treatment paradigm of PBL is emerging.21 A handful of case reports describe the activity of the anti-CD38 monoclonal antibody daratumumab, the anti-CD30 antibody-drug conjugate brentuximab vedotin, and immunomodulatory agents such as lenalidomide.22-25 Although there is a single case report of the successful use of B-cell maturation antigen–directed chimeric-antigen receptor T cells for a patient with refractory disease, there are no data regarding the use of CD19-directed chimeric-antigen receptor T cells in PBL.26 Further exploration into the role of these treatments in combating PBL is merited. Our study suggests that international, multicenter collaboration in PBL can potentially produce the necessary patient numbers for prospective research.
There are some limitations to our study, principally owing to its retrospective nature, and therefore the risk of ascertainment bias and missing data. Nevertheless, the data collected in this study are, overall, granular and comprehensive. Higher rates of missing data were noted for some characteristics, including MYC rearrangement and both CD19 and CD30 status. Regarding MYC status, in the vast majority of missing data were owing to this testing not being conducted, rather than incomplete data reporting.
Missing data were handled in our study by inclusion in the analysis as a separate category. To assess for bias, an additional complete case analysis was performed, in which only patients with complete data were analyzed (supplemental Table 3). The effect sizes from the complete case analysis were similar to our reported findings, suggesting that grouping missing data as a separate category was an appropriate method. Heterogeneity in baseline patient features also introduces confounding bias, which limits the generalizability of the findings, particularly those relating to the effectiveness of different treatment approaches.
Finally, we were unable to conduct a centralized pathology review, which is frequently the case in large, multicenter, real-world studies. However, each contributing site was a major hematology tertiary referral center, with robust internal review processes and pathologists highly experienced in lymphoma diagnosis. Consistent and specific diagnostic criteria for PBL were applied, in keeping with the contemporary World Health Organization classification, to ensure consistent inclusion criteria across participating sites.4 Reassuringly, histological discordance rates between plasma cell neoplasms and lymphomas are in practice likely low.27,28
This study has a number of key strengths. To our knowledge, this is the largest single cohort study of PBL, a rare disease described in the literature to date, pooling data from a large number of centers across 4 countries. The next largest single-patient series reported outcomes of 135 patients from 2 European countries.9 The remainder of the published evidence draws from small to modest-sized series, registry data with limited patient information, or literature reviews of previously published cases.7,8,11,29-31 The relatively large size of this study enabled more robust statistical analysis of survival risk factors. Furthermore, the inclusion of patients from multiple centers provided a more diverse population, which enhances the generalizability of the findings.
Conclusions
PBL remains a relatively rare and challenging entity, with poor long-term outcomes and no standardized approaches to treatment. Future research is warranted and may include prospective studies of different regimens, incorporation of novel therapies, such as immunotherapies and targeted agents, and exploration of tailored approaches based on specific clinical, virological, or molecular features.
Acknowledgments
The authors thank the health care professionals and staff at the participating centers for their contributions as well as the patients from whom these data were derived. This study was coordinated by investigators from the Australasian Lymphoma Alliance.
The contribution from the University of York for this study was supported by Cancer Research UK, grant number 29685, and Blood Cancer UK, grant number 1503, which provides funding for the Haematological Malignancy Research Network, United Kingdom. P.R.D.C. was awarded an American Society of Hematology Abstract Achievement Award in 2020 for this study.
Authorship
Contribution: P.R.D.C. drafted the manuscript, collated the data, contributed to data analysis and interpretation, and reviewed and edited the manuscript; A.J. performed statistical analysis, drafted the manuscript, and reviewed and edited the manuscript; K.C., A.S.G., C.B., M.B., J.K., S. Milliken, P.M., C.P.F., S. Montoto, W.O., G.P.C., K.M., K.M.L., S.I., S.K., M.P.L., D.K., N.W.D., A.-M.W., P.F., C.K.Y., S.H., M.K., L.H.S., A.S., H.R., A.M., Q.L., R.D., G.F., K.P., D.P., N.T., and K.W.S. provided the data, contributed to data analysis and interpretation, and reviewed and edited the manuscript; M.N.P. conceived the study, contributed to the data analysis and interpretation, and reviewed and edited the manuscript; and N.H. conceived and coordinated the study, provided data, contributed to the data analysis and interpretation, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pietro R. Di Ciaccio, Department of Haematology, The Canberra Hospital, Bldg 19, Level 5, The Canberra, Yamba Dr, Garran, ACT 2605, Australia; email: pietro.diciaccio@act.gov.au; and Nada Hamad, Department of Haematology, The Kinghorn Cancer Centre, 370 Victoria Street, Darlinghurst, NSW 2010, Australia; email: nada.hamad@svha.org.au.
References
Author notes
An earlier iteration of this study was presented as an oral abstract at the 62nd annual meeting of the American Society of Hematology, virtual, 5–8 December 2020.
Original data and deidentified patient data are available on request from corresponding author Pietro R. Di Ciaccio (pietro.diciaccio@act.gov.au).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

Comments
Response to Dr. Venkataraman
Similarly for CNS involvement, the p-value provided for in Table 1 is a test of equality of proportions and not two-way pair wise comparison, as there are more than two categories (“not-tested/unknown” values were included). PI-containing regimens were not reported as significantly different amongst IS and NIS patients in our analysis.
The proportion of oropharyngeal tumor location among HIV positive patients was 29% versus 31% amongst HIV negative patients.
CD20