We evaluated the cost-effectiveness of prophylaxis with recombinant von Willebrand factor (rVWF) vs with plasma-derived von Willebrand factor (pdVWF) for patients with severe Von Willebrand disease. We found that rVWF is a cost-saving factor replacement compared with pdVWF across all willingness-to-pay thresholds in the United States.
TO THE EDITOR:
von Willebrand disease (VWD) is the most common inherited bleeding disorder and exhibits significant variability in bleeding phenotype across patients.1,2 International guidelines conditionally recommend long-term prophylaxis to reduce bleeding events for patients with severe and frequent bleeding phenotypes.3 At the time of this conditional recommendation, only 10% of patients with severe VWD are treated prophylactically, unlike patients with severe hemophilia (∼45%).4 In the United States, there are currently 2 approved classes of factor replacement therapy: plasma-derived von Willebrand factor (pdVWF) and recombinant von Willebrand factor (rVWF). The cost-effectiveness of prophylaxis options for patients with severe VWD in the United States is unknown.
To fill this gap, we built a Markov cohort of adult patients with severe VWD to evaluate the cost-effectiveness of prophylaxis with rVWF vs pdVWF from a US health care system perspective. Monthly transition probabilities for patients’ breakthrough bleeding while on pdVWF and rVWF were 0.0416 and 0.0231, respectively, and were derived from an open-label, prospective, phase 3 study employing a pdVWF vs rVWF switch design for patients with severe VWD phenotypes, with a median start age of 34 years (supplemental Table 1; available on the Blood website).5 Costs of prophylaxis and VWD-specific breakthrough bleed health resource use in the United States were sourced from previously published literature and the Centers for Medicare & Medicaid Services, accounting for bleed category, severity, and bleed-specific mortality. pdVWF and rVWF product dosing were sourced from the US Food and Drug Administration package inserts (see the supplemental Data for product dosing specifics).6-8 Utility weights for patients with severe VWD on prophylaxis (ie, utilities in both strategies) were drawn from EuroQol 5-dimension (EQ-5D)–specific utility data from patients with severe hemophilia on prophylaxis and further tested with extensive sensitivity analyses (supplemental Table 1).9 Costs and utilities were discounted by 3% annually with a monthly cycle length to best reflect bleed frequency.10,11
The primary outcome was the incremental cost-effectiveness ratio or the incremental net monetary benefit if the intervention (rVWF) was found to be cost saving, reported over a lifetime time horizon across a range of accepted willingness-to-pay thresholds in the United States. Secondary outcomes were threshold analyses for the (1) breakthrough bleed rate, (2) frequency of infusions, and (3) product cost ratio (rVWF/pdVWF) necessary for the nonfavored product to become favored. Additional scenario analyses examined whether (1) nonsignificance in the point estimate of bleed reduction with rVWF in the phase 3 study (ie, assuming rVWF leads to no decrease in bleed events vs pdVWF), (2) management of major bleeding events with rVWF and recombinant factor VIII (rFVIII), or (3) a nonlifetime time horizon (ie, 5 years) affects the result (ie, which strategy is cost-effective). Deterministic and probabilistic sensitivity analyses are described in the supplemental Data.
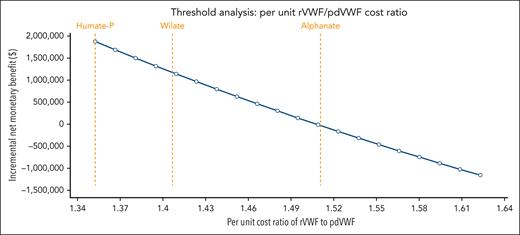
In the base case, treatment with prophylactic pdVWF and rVWF yielded discounted lifetime costs of $18.9 million and $17 million at discounted quality-adjusted life years of 22.5 for both. The incremental net monetary benefit across US willingness-to-pay thresholds was $1.88 million (95% credible interval, $1.6 million-$2.2 million), with rVWF favored in 100% of 10 000 Monte Carlo iterations across probabilistic sensitivity analyses in base-case and scenario analyses (Table 1). Deterministic sensitivity analysis showed that the model was sensitive to weekly prophylaxis product cost (ie, frequency and cost of factor prophylaxis) and not to breakthrough bleed treatment cost or utility weights (supplemental Figure 1). Threshold analyses revealed that for pdVWF to be favored, the (1) bleed rate on pdVWF prophylaxis would have to be >55% lower; (2) average weekly pdVWF and rVWF infusion frequencies would need to decrease to 2.7 and increase to 2.23, respectively; and (3) rVWF/pdVWF per-unit cost ratio to be >1.51.
Base-case and probabilistic sensitivity analyses for rVWF vs pdVWF prophylaxis across all accepted WTP thresholds in the United States
| Prophylactic treatment . | Cost (95% credible interval), million USD . | Effectiveness (95% credible interval), QALY . | % Cost-effective at WTP $50 000/QALY . | % Cost-effective at WTP $100 000/QALY . | % Cost-effective at WTP $150 000/QALY . |
|---|---|---|---|---|---|
| rVWF | 17.0 (16.6-17.6) | 22.5 (21.1-23.9) | 100 | 100 | 100 |
| pdVWF | 18.9 (18.5-19.5) | 22.5 (21.1-23.9) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $1 880 000 ($1 560 000-$2 190 000) | |||||
| Scenario analysis: significant bleed reduction with rVWF | |||||
| rVWF | 16.9 (16.6-17.3) | 22.5 (21.1-23.9) | 100 | 100 | 100 |
| pdVWF | 18.9 (18.5-19.5) | 22.5 (21.1-23.9) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $2 020 000 ($1 350 000-$2 790 000) | |||||
| Scenario analysis: breakthrough bleed management with rFVIII | |||||
| rVWF | 17.0 (16.6-17.7) | 22.5 (21.1-23.9) | 100 | 100 | 100 |
| pdVWF | 18.9 (18.5-19.5) | 22.5 (21.1-23.9) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $1 840 000 ($1 520 000-$2 160 000) | |||||
| Scenario analysis: 5-ytime horizon | |||||
| rVWF | 3.3 (3.2-3.5) | 4.4 (4.1-4.7) | 100 | 100 | 100 |
| pdVWF | 3.7 (3.6-3.8) | 4.4 (4.1-4.7) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $370 000 ($310 000-$430 000) | |||||
| Prophylactic treatment . | Cost (95% credible interval), million USD . | Effectiveness (95% credible interval), QALY . | % Cost-effective at WTP $50 000/QALY . | % Cost-effective at WTP $100 000/QALY . | % Cost-effective at WTP $150 000/QALY . |
|---|---|---|---|---|---|
| rVWF | 17.0 (16.6-17.6) | 22.5 (21.1-23.9) | 100 | 100 | 100 |
| pdVWF | 18.9 (18.5-19.5) | 22.5 (21.1-23.9) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $1 880 000 ($1 560 000-$2 190 000) | |||||
| Scenario analysis: significant bleed reduction with rVWF | |||||
| rVWF | 16.9 (16.6-17.3) | 22.5 (21.1-23.9) | 100 | 100 | 100 |
| pdVWF | 18.9 (18.5-19.5) | 22.5 (21.1-23.9) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $2 020 000 ($1 350 000-$2 790 000) | |||||
| Scenario analysis: breakthrough bleed management with rFVIII | |||||
| rVWF | 17.0 (16.6-17.7) | 22.5 (21.1-23.9) | 100 | 100 | 100 |
| pdVWF | 18.9 (18.5-19.5) | 22.5 (21.1-23.9) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $1 840 000 ($1 520 000-$2 160 000) | |||||
| Scenario analysis: 5-ytime horizon | |||||
| rVWF | 3.3 (3.2-3.5) | 4.4 (4.1-4.7) | 100 | 100 | 100 |
| pdVWF | 3.7 (3.6-3.8) | 4.4 (4.1-4.7) | 0 | 0 | 0 |
| Incremental net monetary benefit at WTP $50 000-$150 000/QALY: $370 000 ($310 000-$430 000) | |||||
All point estimates rounded to maximum 3 significant digits.
QALY, quality-adjusted life year; rFVIII, recombinant factor VIII; USD, US dollar; WTP, willingness to pay.
rVWF and pdVWF are the currently approved classes of VWF replacement therapy in the United States and differ largely in composition and half-life. rVWF lacks factor VIII, contains more high-molecular-weight multimers, and has a half-life that is 1.4 times longer than pdVWF: a difference that contributes to its cost-saving benefit over pdVWF.12-14 Maintaining infusion-based prophylaxis is known to pose a significant burden for patients because of repeated venipuncture, the associated time burden, and monetary costs. These challenges have created obstacles for sustained use of prophylactic factor treatment in hemophilia A, leading to nonadherence and exposing patients to heightened risks of severe bleeding.15 A previous study showed that for patients requiring long-term prophylaxis for hemophilia treatment, the frequency of infusions was the most important factor when choosing their prophylactic treatment.16 The same considerations are at play (and understudied compared with hemophilia A) for patients with severe VWD.
In extensive sensitivity analyses, we show that the cost of prophylaxis, a product of factor cost per unit and number of weekly infusions—and not the cost of managing a bleeding event—determines which strategy is cost-effective. These parameters should be included in future studies on VWF prophylaxis. A prior review comprehensively outlined the dosing regimen and frequency of prophylaxis for VWD, indicating that most factor replacement therapies are administered 2 to 3 times per week.17 Our threshold analyses revealed that for patients with VWD who are on pdVWF twice weekly for >70% of a given year, remaining on pdVWF the following year maximizes accrued health benefit for treatment cost invested. For patients who require >2.7 pdVWF weekly infusions on average, rVWF is the cost-saving choice.
Prophylaxis with rVWF is cost saving specifically compared with the most used pdVWF products in the United States: Humate-P and Wilate (Figure 1).18 Per-unit Alphanate is notably cheaper and—although not indicated in patients with severe VWD undergoing major surgery—the threshold cost ratio favoring pdVWF over rVWF of 1.51 coincidentally aligns exactly with Alphanate (Figure 1). Historically, Humate-P became favored in the 1980s to 1990s because of the presence of more higher-molecular-weight multimers (than other pdVWF concentrates) and the pasteurization process when the field was reeling from the hepatitis C and HIV epidemics.19 Wilate was developed in an attempt to have a product with higher specific activity, better preserved triplet multimer structure, additional virucidal methods, and more physiologic VWF:ristocetin cofactor/FVIII ratios. Clinical use at US institutions between the 3 in 2024 is driven by formulary availability.
Threshold analysis for per-unit rVWF/pdVWF cost ratio necessary to favor pdVWF with labeled corresponding thresholds for Humate-P, Wilate, and Alphanate. All point estimates rounded to maximum of 3 significant digits.
Threshold analysis for per-unit rVWF/pdVWF cost ratio necessary to favor pdVWF with labeled corresponding thresholds for Humate-P, Wilate, and Alphanate. All point estimates rounded to maximum of 3 significant digits.
Study strengths of this industry influence-free economic analysis include basing our analysis on prospective, phase 3 study data that used an intrapatient comparison switch design that effectively minimizes confounding (except for intratrial, within-patient secular trend), accounting for VWD- and severity-specific bleeding cost, and derivation of nonnormative clinical thresholds and cost ratios that facilitate independent formulary decision-making across available factor products to each institution. This study is limited by the assumption that quality of life in patients with severe VWD on prophylaxis is the same as that in patients with severe hemophilia A on prophylaxis, a necessity because of the lack of available utility weights in VWD. However, we thoroughly address this limitation in our sensitivity analyses and additionally note that, given our comparison of 2 strategies in which both patient populations remain on prophylaxis (vs on demand), the same quality-adjusted life expectancy negates quality of life as a variable that can change our conclusion. Furthermore, if bleed reduction with rVWF is assumed to be significant (ie, scenario analysis), then the pdVWF prophylaxis strategy costs more and reduces quality-adjusted life expectancy (Table 1), again supporting the conclusion that rVWF is cost-effective regardless of utility weights, further supported in deterministic sensitivity analyses (supplemental Figure 1).
In summary, we evaluated the cost-effectiveness of rVWF vs pdVWF prophylaxis treatment options for patients with severe VWD. At current pricing in the United States, prophylactic rVWF is cost saving for patients averaging >2.7 weekly pdVWF infusions. A per-unit rVWF/pdVWF cost ratio of >1.51 is the critical threshold for a given pdVWF product to become favored over rVWF.
Acknowledgments
G.G. is supported by The Frederick A. DeLuca Foundation and the Yale Bunker Endowment.
The funders had no role in the identification, design, conduct, or reporting of the analysis.
Authorship
Contribution: C.W., S.I., A.C., and G.G. designed the study; and all authors wrote and edited the manuscript.
Conflict-of-interest disclosure: A.C. has served as a consultant for MingSight, New York Blood Center, Pfizer, Sanofi, and Synergy; and has received authorship royalties from UpToDate. The remaining authors declare no competing financial interests.
Correspondence: George Goshua, Yale School of Medicine, 333 Cedar St, New Haven, CT 06520; email: george.goshua@yale.edu.
References
Author notes
Original data are available on request from the corresponding author, George Goshua (george.goshua@yale.edu).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal