Visual Abstract
Neutrophils are the first migrating responders to sterile and infectious inflammation and act in a powerful but nonspecific fashion to kill a wide variety of pathogens. It is now apparent that they can also act in a highly discriminating fashion; this is particularly evident in their interactions with other cells of the immune system. It is clear that neutrophils are present during the adaptive immune response, interacting with T cells in complex ways that differ between tissue types and disease state. One of the ways in which this interaction is mediated is by neutrophil expression of HLA molecules and presentation of antigen to T cells. In mice, this is well established to occur with both CD4+ and CD8+ T cells. However, the evidence is less strong with human cells. Here, we assembled available evidence for human neutrophil antigen presentation. We find that the human cells are clearly able to upregulate HLA-DR and costimulatory molecules; are able to process protein antigen into fragments recognized by T cells; are able to enter lymph node T cell zones; and, in vitro, are able to present antigen to memory T cells, inducing proliferation and cytokine production. However, many questions remain, particularly concerning whether the cell-cell interactions can last for sufficient time to trigger naïve T cells. These experiments are now critical as we unravel the complex interactions between these cells and their importance for the development of human immunity.
Introduction
Traditionally, neutrophils have been considered terminally differentiated, short-lived cells that have little impact on adaptive immunity. However, we now appreciate them to be cells that survive longer than previously thought,1 are present in lymph nodes and lymphatics,2-6 and exist in tissues long term during chronic inflammation.7,8 These facts mean that they are present at the same time as developing T-cell responses. Interactions between T cells and neutrophils have been frequently described in the literature; these interactions result in profound alterations in the function of the T cells.1,9-12 A summary of interactions between neutrophils and T cells is shown in Figure 1. In addition, there are multiple studies observing that mouse neutrophils can upregulate MHC class II molecules4,5,13-15 and transport antigen to sites of T-cell priming.2 In vitro, using primed isolated cells, murine neutrophils have been shown to present antigen to both CD4+ and CD8+ T cells.14,16-20
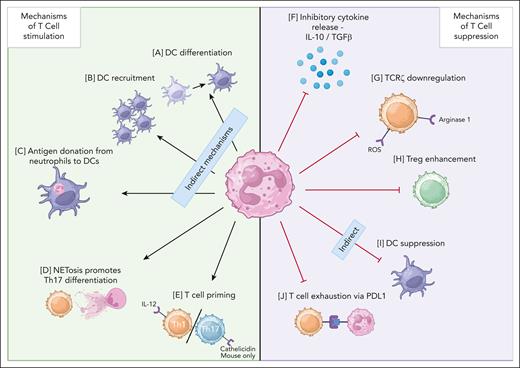
Mechanisms of T-cell and neutrophil interactions. (A) DC differentiation72,73 (activation). (B) DC recruitment72,74 (activation). (C) Antigen donation from neutrophils to DCs75,76 (activation). (D) NETosis induces Th17 differentiation9,77 (activation). (E) T-cell priming of Th1 and Th17 T cells9,78,79 (activation). (F) Inhibitory cytokine release80-83 (suppression). (G) TCRζ downregulation84-87 (suppression). (H) Treg enhancement88,89 (suppression). (I) DC suppression11,90 (suppression). (J) T-cell exhaustion91,92 (suppression). Cathelicidin’s impact on the Th17/Th1 balance has only been demonstrated in mouse cells as of 2024 and is therefore labeled “mouse only.” IL-10, interleukin-10; NET, neutrophil extracellular trap; TGFβ, transforming growth factor beta; Th, T helper cell; TCR, T-cell receptor; DC, dendritic cell. Figure made with Biorender.com.
Mechanisms of T-cell and neutrophil interactions. (A) DC differentiation72,73 (activation). (B) DC recruitment72,74 (activation). (C) Antigen donation from neutrophils to DCs75,76 (activation). (D) NETosis induces Th17 differentiation9,77 (activation). (E) T-cell priming of Th1 and Th17 T cells9,78,79 (activation). (F) Inhibitory cytokine release80-83 (suppression). (G) TCRζ downregulation84-87 (suppression). (H) Treg enhancement88,89 (suppression). (I) DC suppression11,90 (suppression). (J) T-cell exhaustion91,92 (suppression). Cathelicidin’s impact on the Th17/Th1 balance has only been demonstrated in mouse cells as of 2024 and is therefore labeled “mouse only.” IL-10, interleukin-10; NET, neutrophil extracellular trap; TGFβ, transforming growth factor beta; Th, T helper cell; TCR, T-cell receptor; DC, dendritic cell. Figure made with Biorender.com.
We are interested in examining whether the collection, processing, and presentation of antigen to T cells occurs in vivo during human inflammatory disease. Murine and human neutrophils are very different (reviewed by Eruslanov et al21), and many of the previous studies were performed in mice. Despite this, many papers state that neutrophil antigen presentation occurs, extrapolating the murine data to human cells. In light of this, here we examine the evidence for antigen presentation in vivo by human neutrophils. We ask a series of questions to evaluate the evidence for this phenomenon.
Can human neutrophils be induced to express markers of antigen presentation?
In normal conditions, human neutrophils circulating in the blood do not express cell surface markers related to antigen presentation, such as the human leukocyte antigen (HLA) complex or the costimulatory molecules CD80 or CD86.19,22,23 They can, however, can be induced to express these molecules in vitro when stimulated with inflammatory mediators (summarized in Figure 2).24,25 In particular, treatment with 30 ng/ml interferon gamma (IFN-γ) causes neutrophil upregulation of class II major histocompatibility complex transactivator, the master regulator of HLA molecule expression.26 The expression of these markers of antigen presentation has been thoroughly reviewed previously.27
Mechanisms through which HLA-DR is expressed on human neutrophils. (A) DAMPs: ox-LDL and E-CIRP.15 (B) Phagocytosis of red blood cells.23 (C) Cytokines: GM-CSF and IFN-γ.24,25 DAMPs, damage-associated molecular patterns; E-CIRP, extracellular cold-inducible RNA-binding protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; ox-LDL, oxidized low-density lipoprotein; RBC, red blood cells. Figure made with Biorender.com.
Mechanisms through which HLA-DR is expressed on human neutrophils. (A) DAMPs: ox-LDL and E-CIRP.15 (B) Phagocytosis of red blood cells.23 (C) Cytokines: GM-CSF and IFN-γ.24,25 DAMPs, damage-associated molecular patterns; E-CIRP, extracellular cold-inducible RNA-binding protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; ox-LDL, oxidized low-density lipoprotein; RBC, red blood cells. Figure made with Biorender.com.
Neutrophils can be primed by IFN-γ for future reactive oxygen species (ROS) production. Interestingly, patients with genetic defects that cause a loss in the activity of nicotinamide adenine dinucleotide phosphate (NADPH oxidase), a major pathway for ROS production, show suppressed induction of the HLA-DR complex in the presence of GM-CSF and IFN-γ in vitro.28,29 Blockade of NADPH in prolonged cultures of healthy control neutrophils also leads to a loss in the expression of the class II molecules. This highlights the role that intracellular ROS plays in the cytokine-mediated expression of class II antigen presentation markers.28
Presentation of antigen to T cells must be followed by costimulation to prevent the initiation of anergy. Again, ex vivo peripheral blood neutrophils do not express costimulatory molecules, but they are able to translocate intracellular stores of such molecules, such as CD80 and CD86, to their cellular surface after crosslinking of Mac-1.30 Intracellular stores of CD80 and CD86 appear to be present in all healthy donor neutrophils.31 Therefore, we conclude that human neutrophils are capable of expressing molecules necessary for the presentation of antigen.
Is this expression seen in vivo?
Next, we asked whether neutrophils isolated and analysed directly ex vivo have been shown to express these molecules, rather than examining cells treated with cytokines in vitro, as the studies mentioned previously demonstrated that ex vivo peripheral blood neutrophils do not do so.
In patients with rheumatoid arthritis, expression of HLA class II molecules is noted on neutrophils present in the inflamed joint.32 RNA sequencing analysis of neutrophils isolated from synovial fluid demonstrated that neutrophils in the synovium have higher expression of the HLA isotype HLA-DR.32,33 Furthermore, when neutrophils taken from healthy donors were exposed to synovial fluid from patients with rheumatoid arthritis, upregulation of HLA class II occurred32,33; this is proposed to be induced by the high physiological concentrations of IFN-γ (650 pg/ml) and GM-CSF (1 ng/ml) that were released by activated T cells present at that site.33 This is supported by evidence gathered after the instillation of IFN-γ for the treatment of chronic granulomatous disease and osteopetrosis,34 which does elicit HLA-DR expression on circulating neutrophils; however, interestingly, only ∼50% of the cells respond in this way.34 This increased expression of HLA-DR was linked to posttranscriptional changes rather than transcriptional regulation, because HLA-DR messenger RNA expression was identified in all neutrophils from both groups.34
It is clear therefore that neutrophils can express class II molecules in vivo during inflammatory states. There is also evidence that this expression may occur in steady state in tissues other than peripheral blood. Importantly, neutrophils within lymph nodes from a variety of anatomical locations express HLA-DR.35 This is variable between donors, with 10% to 50% of neutrophils showing expression. Other examples of tissue neutrophil expression of HLA in healthy donors are rare, although this may reflect a lack of sampling or suboptimal tissue preparation methods, which lead to the undercounting or death of neutrophils.
A model in which grass allergen is introduced into the skin followed by blister creation demonstrated that between 10% and 25% of blister neutrophils expressed HLA-DR within 24 hours, but no peripheral blood neutrophils in the same donors were HLA-DR+.36 Finally, HLA-DR+ neutrophils travel in the blood of patients with visceral leishmaniasis37; these HLA-DR+ cells also expressed CD80 and CD86.
Neutrophils in vivo can, therefore, express the molecules required to present to T cells. The mechanisms through which this occurs appear often to be linked to IFN-γ (Figure 2). Neutrophils freshly isolated from peripheral blood upregulate HLA-DR and costimulatory molecules CD86, CD80, CD40, and CD83 in the presence of antigen-specific memory T cells; interestingly, naïve T cells did not have the same effect.18 This could be explained by the fact that memory T cells produce IFN-γ in a higher abundance than naïve T cells and produce this more readily from a smaller stimulus.38-40 Because inflamed tissues contain IFN-γ–producing memory T cells, this indicates that neutrophils have the potential to respond to a wide range of stimuli at such sites, leading to the induction of antigen presenting markers.15,40,41
Other mechanisms are also proposed. Various studies have found tumor-associated neutrophils that express HLA-DR and CD86.6,19,20,42 Expression of these markers was also seen in distant tissue neutrophils but not to the same extent and were not seen at all in peripheral blood neutrophils, further demonstrating alterations in neutrophil phenotype between tissue sites.6,17,36,37,43 The expression of HLA-DR on tumor-associated neutrophils varies between different cancer types, with adenocarcinoma having a significantly higher proportion of HLA-DR+ neutrophils than squamous cell carcinoma.44 In addition, in tumors >3 cm in diameter, the number of HLA-DR–expressing neutrophils is greatly reduced. This is further supported by RNA sequencing data that show neutrophils moving from circulation into the tumor environment initially express HLA-DR.20,44 However, as they progress further into the tumor, there is a shift away from this phenotype.19,20,44 This change in phenotype was linked to the hypoxia inducible factor HIF-1α, because larger tumors tend to be more hypoxic, and after inducing HIF-1α in vitro, the number of HLA-DR expressing neutrophils was significantly decreased.20
Can human neutrophils process protein antigen and load it onto HLA molecules?
Therefore, neutrophils can produce extracellular markers of antigen presentation, but to actually present to T cells in vivo, they also need to process proteins into peptides and load them onto the HLA complex.
Neutrophil proteases within lysosomes are very effective at breaking down antigens. This occurs within minutes, producing detectable peptides suitable for HLA loading that match frequently recognized T-cell activation regions. In 1 study, it was found that after 2 hours of this, there was no detectable intact antigen remaining36; in contrast, using monocytic proteases, 10% of intact antigen was still detectable after 24 hours.36,43 This highlights the key ability of neutrophil proteases to break down antigens into peptides suitable for antigen presentation. In support of this, Pylaeva et al6 demonstrated using the fluorescent DQ-ovalbumin system that tumor-isolated neutrophils from patients with head and neck cancer were able to process antigen efficiently, and Davey et al showed processing and loading of the influenza M1 protein onto neutrophil HLA molecules.45
It is worth considering that the ability of neutrophils to rapidly break down antigen, compared with traditional antigen-presenting cells (APCs), may be 1 reason why neutrophils have been repeatedly shown to be incapable of inducing the same strength of T-cell activation as those APCs; antigen that is more readily degraded in the lysosome persists for a shorter length of time and elicits a reduced antibody response 46 and T-cell proliferation.23,47
To prevent early or incorrect antigens binding to the HLA-DR complex, the class II invariant chain peptide (CD74) binds to HLA-DR, helping prevent premature binding. This molecule, which regulates the correct binding and presentation of antigens, is actively expressed in human neutrophils.26 The release of the class II invariant chain peptide from the HLA-DR molecule is then mediated by HLA-DM, allowing the antigenic peptide to bind to the target. HLA-DM is expressed in human neutrophils but is not present when the cells are negative for HLA-DR. Both proteins are key regulators of antigenic peptide loading on HLA-DR.26,36
Taking this evidence together, we conclude that it is likely neutrophils can process and present antigen to T cells, because they express the classical HLA complex and costimulatory molecules, as well as key molecules that are important to correct antigenic peptide loading.
Are neutrophils present in human lymph nodes?
Whether neutrophils can present antigen to memory T cells in tissues or to naïve T cells in lymph nodes is 2 separate questions. For presentation to naïve T cells, it is necessary that the neutrophils be present in lymph nodes. In mice, this has been observed after vaccination,2,3,9,48 in autoimmune disease,5,49 in tumor-bearing animals,50 and during infection.51-53
In humans, neutrophils traffic to the inguinal, mesenteric, and thoracic lymph nodes during steady state.35 Murine neutrophils traffic into lymph nodes predominantly via high endothelial venules but also via afferent lymphatics, and move to the interfollicular zone in which CD4+ T cells reside.35 It is hypothesized that this may hold true for human neutrophils, because they were observed close to high endothelial venules and in the node interstitium. However, this needs to be validated with further studies.
Neutrophils are present in nonmetastatic and metastatic lymph nodes in patients with a variety of cancers.6,54-56 These neutrophils are in close proximity to T cells (<1 cell diameter apart). It was found that depending on the stage and presence of metastasis within the lymph node, neutrophils had either stimulatory (linked with upregulation of antigen presentation markers) or suppressive effects.6 This highlights our need to better understand the function of neutrophils within the human lymph node, because this is currently not well defined. We may be able to resolve these questions by adapting techniques to neutrophils that have previously been used to characterize the role of other immune cells within the lymph node.4,6,57,58
Do extended interactions of neutrophils and T cells occur?
Naïve T cells require extended contact with APCs for productive triggering of the T-cell receptor to occur.59-61 This involves formation of an immune synapse, a stable interaction between T cells and APCs triggered by binding of the T-cell receptor to HLA molecules, followed by clustering of adhesion and costimulatory molecules at the interface between the cells. The synapse allows for concentration and productive binding of signaling molecules, passage of soluble mediators, and sustained productive signaling in the T cell.
Attachments between T cells and suppressive neutrophils isolated from patients with sepsis have been observed; these are dependent on the integrin CD11b. Close interactions have been demonstrated for cells from healthy donors6 but have not yet been seen for sustained periods of time. Whether neutrophils and T cells do form a ‘synapse’ in the sense we use this term for a traditional APC–T-cell interface is unclear. We are unaware of any studies that have demonstrated concentration of integrin and signaling molecules at the cell-cell interaction point, for example. However, neutrophils expressing the integrin ICAM-1 are seen in patients, such as during infection with respiratory syncytial virus or during sepsis.62,63 ICAM-1 expression coincides with phagocytosis64; this is of importance as phagocytosis would be relied upon for neutrophils to process antigens into presentable antigenic peptides. Finally, 1 study demonstrated partial dependence of neutrophil antigen presentation and subsequent T-cell proliferation on ICAM1.33 In addition, neutrophils have been observed to form synapse-like structures with other cells,65,66 suggesting this may also occur at the T-cell interaction point also.
Have any isolated human neutrophils been shown to present antigen and induce T-cell proliferation?
We finally questioned what evidence there is of human neutrophils presenting antigens to T cells directly and inducing their proliferation in the absence of anti-CD3 or anti-CD28 antibodies in the cell culture. In our literature search, we found a number of papers that had examined proliferation of T cells incubated with human neutrophils without polyclonal stimulation of the T cells (summarized in Table 1).
Analysis of experiments testing direct neutrophil presentation or crosspresentation of antigen, using human cells, in the absence of anti-CD3 or anti-CD28 antibodies
| First author . | Reference . | Antigen used . | T cells used . | Neutrophils used . | Results . |
|---|---|---|---|---|---|
| Fanger | 14 | Superantigen and tetanus toxoid | Tetanus-toxoid–specific CD4+ T cells expanded from a healthy donor | Stimulated for 24 h with IFN-γ and GM-CSF and then irradiated | T-cell proliferation in response to superantigen but not tetanus toxoid (protein or peptide) |
| Vono | 18 | CMV antigen pp65 and influenza virus HA | Primary CD4+ T cells from seropositive donors | Directly ex vivo (pulsed 1 hour with antigen) | Proliferation and cytokine production by T cells, which is inhibited by anti–HLA-DR antibodies |
| Meinderts | 23 | Tetanus toxoid | Directly ex vivo CD4+ and CD8+ T cells | Incubated ± opsonized red blood cells. Second population of neutrophils given to T cells on d 4 of culture | Proliferation and cytokine production of T cells. This was inhibited by anti-HLA-DR antibodies. Only CD4+ and not CD8+ T cells proliferated |
| Radsak | 25 | Superantigen Staphylococcus enterotoxin E and tetanus toxoid | Tetanus-toxoid–specific CD4+ T cell lines expanded from healthy donors | Treated for 24 h with IFN-γ and GM-CSF | Proliferation of T cells in response to superantigen and to tetanus toxoid, inhibited by anti–HLA-DR and anti-CD86 antibodies. Only CD4+ and not CD8+ cells proliferated. Only autologous and not heterologous neutrophils could stimulate proliferation. |
| Polak | 36 | Birch pollen antigen Bet v 1 | Bet v 1–specific CD4+ T cell lines derived from allergic patients | Stimulated with GM-CSF, IFN-γ, and IL-3 and then irradiated | Proliferation and cytokine production by T cells |
| Sharma | 37 | Soluble Leishmania antigen | Directly ex vivo total CD3+ T cells | Normal-density or low-density neutrophils, directly ex vivo | No proliferation |
| Iking-Konert | 33 | Elastase and tetanus toxoid | CD4+ T cell lines specific for elastase and tetanus toxoid | Treated for 24 h with IFN-γ and GM-CSF | Proliferation by T cells in response to autologous but not heterologous neutrophils with antigen |
| Davey | 45 | Tetanus toxoid and influenza virus M1 matrix protein, as well as superantigen | CD8+ T cell lines and primary peripheral blood mononuclear cells | Cultured for 48 h with Vγ9Vδ2 or MAIT cells, CD3/CD28 stimulation or HMB-PP to activate unconventional T cells or IFNγ, TNF, and/or GMCSF | T-cell proliferation and cytokine production in response to antigen processed and presented by neutrophils. CD8+ T-cell response to exogenous antigen (cross-presented antigen). Dependent on priming of the neutrophils by culture with unconventional T cells. |
| First author . | Reference . | Antigen used . | T cells used . | Neutrophils used . | Results . |
|---|---|---|---|---|---|
| Fanger | 14 | Superantigen and tetanus toxoid | Tetanus-toxoid–specific CD4+ T cells expanded from a healthy donor | Stimulated for 24 h with IFN-γ and GM-CSF and then irradiated | T-cell proliferation in response to superantigen but not tetanus toxoid (protein or peptide) |
| Vono | 18 | CMV antigen pp65 and influenza virus HA | Primary CD4+ T cells from seropositive donors | Directly ex vivo (pulsed 1 hour with antigen) | Proliferation and cytokine production by T cells, which is inhibited by anti–HLA-DR antibodies |
| Meinderts | 23 | Tetanus toxoid | Directly ex vivo CD4+ and CD8+ T cells | Incubated ± opsonized red blood cells. Second population of neutrophils given to T cells on d 4 of culture | Proliferation and cytokine production of T cells. This was inhibited by anti-HLA-DR antibodies. Only CD4+ and not CD8+ T cells proliferated |
| Radsak | 25 | Superantigen Staphylococcus enterotoxin E and tetanus toxoid | Tetanus-toxoid–specific CD4+ T cell lines expanded from healthy donors | Treated for 24 h with IFN-γ and GM-CSF | Proliferation of T cells in response to superantigen and to tetanus toxoid, inhibited by anti–HLA-DR and anti-CD86 antibodies. Only CD4+ and not CD8+ cells proliferated. Only autologous and not heterologous neutrophils could stimulate proliferation. |
| Polak | 36 | Birch pollen antigen Bet v 1 | Bet v 1–specific CD4+ T cell lines derived from allergic patients | Stimulated with GM-CSF, IFN-γ, and IL-3 and then irradiated | Proliferation and cytokine production by T cells |
| Sharma | 37 | Soluble Leishmania antigen | Directly ex vivo total CD3+ T cells | Normal-density or low-density neutrophils, directly ex vivo | No proliferation |
| Iking-Konert | 33 | Elastase and tetanus toxoid | CD4+ T cell lines specific for elastase and tetanus toxoid | Treated for 24 h with IFN-γ and GM-CSF | Proliferation by T cells in response to autologous but not heterologous neutrophils with antigen |
| Davey | 45 | Tetanus toxoid and influenza virus M1 matrix protein, as well as superantigen | CD8+ T cell lines and primary peripheral blood mononuclear cells | Cultured for 48 h with Vγ9Vδ2 or MAIT cells, CD3/CD28 stimulation or HMB-PP to activate unconventional T cells or IFNγ, TNF, and/or GMCSF | T-cell proliferation and cytokine production in response to antigen processed and presented by neutrophils. CD8+ T-cell response to exogenous antigen (cross-presented antigen). Dependent on priming of the neutrophils by culture with unconventional T cells. |
CMV, cytomegalovirus; HMB-PP, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate; IL-3, interleukin 3; TNF, tumor necrosis factor; MAIT, mucosa-associated invariant T cells; HA, hemagglutinin; HLA-DR, human leukocyte antigen, DR isotype.
Fanger et al14 were one of the first groups to show that primed human neutrophils (treated with GM-CSF and IFN-γ) increased the proliferation of CD4+ T cells in the presence of superantigen. It was noted by the researchers that the ability of neutrophils to upregulate antigen presentation markers varied markedly between donors. Also tested in this study was the ability to present tetanus toxoid antigen to antigen-specific CD4+ T cells. However, it was found that neutrophils were unable to induce proliferation under these conditions; in this context, it is important to note that although tetanus toxoid antigen requires intracellular processing before presentation, superantigen does not.
In contrast, other studies have shown that neutrophils are able to present tetanus toxoid protein to T cells. Iking-Konert33 incubated peripheral blood neutrophils with IFN-γ and GM-CSF for 24 hours before they were added to expanded tetanus toxoid-specific T-cell clones. The cultures were supplied with tetanus toxoid protein, and proliferation was measured after 5 days. Proliferation of T cells was observed in the presence of activated neutrophils but not ex vivo resting neutrophils or in control samples. In this experiment, purity of the neutrophil preparation was >99%.
Similarly, another study induced HLA-DR expression in neutrophils through the phagocytosis of immunoglobulin G-opsonized red blood cells. The neutrophils in this case were able to present tetanus toxoid in vitro and significantly increase the proliferation of CD4+ T cells.23 Again, in a different study, neutrophils treated for 24 hours with IFN-γ and GM-CSF induced proliferation in CD4+ T cells when the cocultures were provided with tetanus toxoid. This process was, importantly, blocked by antibodies against HLA-DR and CD86. However, the authors acknowledge 1% contamination of monocytes in their preparation.25
Other examples of antigen presentation in vitro include neutrophils taken from healthy human donors that induced CD4+ T-cell proliferation when cultured with the cytomegalovirus antigen PP65 and antigen-specific memory T cells.18 In this experiment, the neutrophil isolation was 99% pure; therefore, it cannot be ruled out that contaminating dendritic cells (DCs) are present in the 1% of nonneutrophil cells and that this is influencing T-cell proliferation.18 Finally, another study demonstrated that neutrophils (again 99% pure, but only contaminated with eosinophils) could express HLA-DR and induce proliferation of allergen-specific memory T cell clones after stimulation with birch pollen antigen in vitro.36
As noted previously, HLA-DR+ neutrophils are common in the blood of patients with visceral leishmaniasis.37 However, these neutrophils were unable to induce T-cell proliferation in a directly ex vivo culture. It is worth noting that the proliferation of all T cells was measured and not specifically memory or antigen-specific cells. Therefore, increases in antigen-specific CD4+ memory T cells may be masked by a lack of increase in the proliferation of other T cells within the population.
These studies therefore demonstrate the potential of neutrophils to process protein antigen and present it via HLA-DR in a way that induces memory T-cell proliferation. The small amounts of contamination of these cultures may, however, be important; if 1% of the neutrophil culture are DCs or monocytes, this could hugely affect the proliferation of T cells over a 5-day period. Another observation is that only 2 of these papers (see Table 1) used cells directly ex vivo. Of these 2 papers, 1 showed proliferation induced by neutrophils, and 1 showed no response. This is, therefore, something that must be investigated in more detail. A key experiment would be isolating HLA-DR+ neutrophils from inflamed tissue and directly ex vivo demonstrating prolonged contact with, and triggering of, autologous T cells in the presence of protein antigen.
In mice, neutrophils can crosspresent antigen to CD8+ cytotoxic T cells, including even naïve cells.17 Crosspresentation refers to the process by which antigen presenting cells take up exogenous antigen, process it, and present it via MHC class I molecules to CD8+ T cells (rather than loading the antigen onto MHC II molecules, which is the classical pathway for exogenous antigen). Using human cells, we were only able to find 1 paper that examined crosspresentation.45 This paper demonstrated that neutrophils were able to induce proliferation of human responder CD8+ T-cell lines after presentation of the influenza M1 protein. Interestingly, this was substantially dependent on the neutrophils being activated with TNF before being added to T-cell cultures, and neutrophils that were not activated with cytokines before being given antigen were unable to crosspresent.
Discussion
In this report we analyzed existing papers relating to antigen presentation by human neutrophils to determine whether it is likely that this process occurs in vivo during inflammatory disease.
We have found substantial evidence that neutrophils can upregulate HLA and costimulatory molecules and can process and present antigenic peptides. The studies demonstrating this are particularly interesting because they show a large difference in phenotype between peripheral blood neutrophils and those in lymph nodes and inflamed tissues, with the latter expressing far more of these presentation-related molecules on the cell surface. This reminds us how important it is to study cells from the tissue of interest during inflammatory disease, because they are often profoundly different to blood cells from the same donor. If this is not possible, we believe it is worth exploring a comparative study of priming or activation conditions to determine which most accurately produce peripheral blood neutrophils whose phenotype matches those in the inflamed tissues.
Next, we examined whether there is any evidence that neutrophils can present antigen to naïve T cells, inducing their proliferation. HLA-DR+ neutrophils are present in the T-cell zones of human lymph nodes, as they are in mice, and the neutrophils at that site express co-stimulatory molecules. However, in our opinion, it is unlikely that neutrophils will be able to present in vivo in a physiologically important manner to naïve T cells, owing to the prolonged interaction required for this process; there is currently no evidence that these long interactions occur. However, the potential for increased life span of neutrophils under inflammatory conditions means this cannot be ruled out, and investigation into longer-term synapse formation between human neutrophils and T cells of all types is something that would be of great interest.
It is very difficult to assess the presentation of antigen to naïve human T cells, owing to the rarity of each clone in the pool available for experiments. Some protocols do exist for different approaches to perform these experiments, however.67,68 It would be of enormous interest to determine whether human neutrophils are capable of presenting antigen to naïve T-cell populations. We are unaware of any such experiments having been performed to date.
On the balance of evidence, we believe, in contrast, that it is likely that neutrophils can present antigen to reactivate memory T cells in lymphoid or inflamed tissues. The number of neutrophils present, their expression of HLA-DR and costimulatory molecules, and the lower threshold required for the activation of memory cells suggest this is possible. A number of studies have been published demonstrating the capability of neutrophils to present antigen to antigen-specific memory T cells or T-cell lines ex vivo. The caveat that these neutrophil cultures may be contaminated with a small number of DCs or monocytes could, however, be important.
Neutrophil heterogeneity is a fast-moving field, with particular focus currently on low-density cells and tissue cell populations, with a key area of study being their interaction with T cells in the development of cancers and autoimmune disease.69 It is interesting to consider whether some of the differences in antigen presentation that we have discussed are a result of experiments using different neutrophil populations. For example, CD10 is often used as a marker of T-cell–suppressive neutrophil populations, whether they are normal or low density.70 Unfortunately, the experiments showing these cells are T-cell suppressive are typically performed by examining the impact of the neutrophils on T-cell response to anti-CD3/CD28 antibodies, and so we cannot assess antigen presentation in these systems. Isolation of low-density CD10+ and CD10– neutrophils as well as normal density (CD10+) neutrophils and assessing all of their abilities to present antigen would be instructive.
When thinking about neutrophil antigen presentation in vivo within mixed immune cell populations, it is clear that neutrophils do not stimulate T-cell proliferation to the same extent as traditional APCs such as DCs; this has been demonstrated in a number of studies in vitro. DCs are significantly more efficient at inducing proliferation, both at lower cell numbers than neutrophils but also with lower antigen concentrations.17 Monocytes can also induce equivalent T-cell proliferation as neutrophils with only 10% of the cell number, highlighting the effectiveness of monocytes at inducing proliferation compared with neutrophils.14 Reasons for the reduced induction of proliferation by neutrophils are the decreased cell surface expression of HLA molecules on neutrophils compared with similarly activated DCs23 and their faster processing of antigen, which may reduce responses in lymphocytes.36,46 However, neutrophils are able to induce proliferation significantly earlier than other APCs in vitro, which makes sense with faster antigen processing abilities.17 Pairing this information with the fact that neutrophils are some of the first cells moving into lymph nodes and tissues bearing antigen (studies performed in mice2,3), we propose that in vivo neutrophils can provide an early start to the adaptive immune response,2,51 in particular kick-starting the reactivation of memory cell populations.
We do not believe, therefore, that neutrophils would ever supplant DCs in antigen presentation but could support the very early T-cell response. We envisage, for example, the inflamed lung of a patient with viral infection. In this situation, lung-resident memory T cells are reactivated by APCs.71 The sheer number of neutrophils infiltrating the lung or equivalent inflamed tissue means even their substandard antigen presentation may be of enormous value in initiating memory T-cell reactivation very early in an infection.
In the lymph nodes, the situation is less clear. It may be that neutrophils play a valuable but not essential role in lymph node priming of T cells via additional presentation of antigen or production of inflammatory mediators. It may also be that even in the lymph node, the chief beneficiaries of neutrophil priming are memory T cells.
Acknowledgment
This work was funded by a Royal Society Dorothy Hodgkin Fellowship (DH150175; E.G.F.).
Authorship
Contribution: A.M. and E.G.F. wrote and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emily Gwyer Findlay, School of Biological Sciences, University of Southampton, Level D, South Academic Block, Southampton General Hospital, Southampton SO16 6YD, United Kingdom; email: e.gwyerfindlay@soton.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal