In this issue of Blood, Uehata et al demonstrate roles for regnase-1 and regnase-3 (Reg1/3) in the control of hematopoietic stem/progenitor cell (HSPC) fates by restricting myeloid differentiation and promoting lymphoid differentiation.1
Hematopoietic stem cells (HSCs) develop into multipotent progenitors (MPPs) and lineage-restricted progenitors that give rise to all blood cell types. Under steady-state conditions, HSCs maintain blood production by balanced production of myeloid-biased MPP2/MPP3 and lymphoid-biased MPP4.2 Multiple sterile and infection-associated inflammatory stimuli can activate HSCs to overproduce MPP2/3 via upregulation of myeloid-priming transcription factor PU.1 and redirect MPP4 exclusively toward myeloid cell output.3,4 Following acute inflammatory stress, HSCs may recover to full functional potency5 or exhibit long-lasting myeloid skewed differentiation.3,5 Aging and chronic inflammatory conditions are associated with myeloid-biased hematopoiesis, which is thought to promote the systemic inflammation that drives numerous age-related diseases, as well as promote the development of myeloid neoplasms.6 Although cell-intrinsic and extrinsic factors that regulate lineage specification have been described,3 our understanding of these molecular mechanisms is still limited and must be expanded to identify therapeutic interventions that allow reliable control over lineage fate choices.
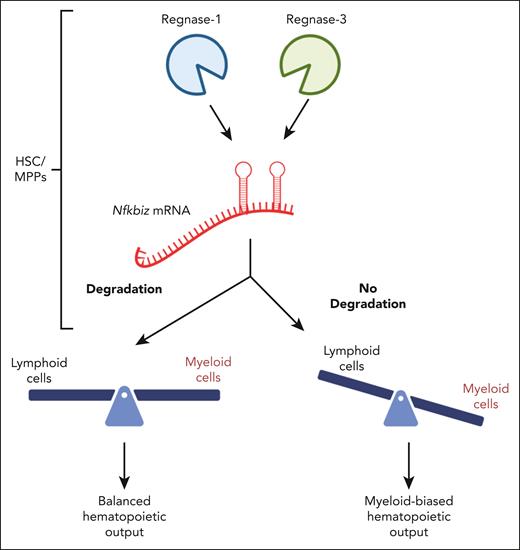
In these studies, the authors demonstrate that Reg1/3 are critical for the control of lineage priming in HSPCs and act to restrict myeloid differentiation and promote lymphoid fates (see figure). Regulatory RNases (regnases) are endoribonucleases that degrade messenger RNAs (mRNAs) harboring stem-loop (SL) structures in their 3’ untranslated regions (UTRs). The authors evaluated Reg1–/– and Reg3–/– mice since these 2 family members are highly expressed in hematopoietic progenitors. Although double-knockout (DKO) mice showed a perinatal lethal phenotype, Reg1–/–, and Reg3–/– mice did not. To evaluate the effects of Reg1 and Reg3 on hematopoiesis, the authors transplanted fetal liver (FL) cells from Reg1–/–, Reg3–/–, or DKO mice. Cells from DKO mice gave rise to markedly decreased B cells and increased CD11b+ myeloid cells, but mice transplanted with Reg1–/–, and Reg3–/– FL cells did not exhibit significant changes in cell composition. Inducible deletion of Reg1/3 in adult mice resulted in a reduction in B cells and an increase in myeloid cells, and deletion of Reg1/3 in lymphoid progenitors using an Il7r-driver Cre resulted in no change in B-cell differentiation. This latter finding confirms that Reg1/3 regulate B-cell development in immature progenitors upstream of common lymphoid progenitors. Single-cell RNA-sequencing evaluation of Lin-Sca-1+c-Kit+ cells from DKO mice revealed enrichment of myeloid and megakaryocytic-erythroid progenitors as well as a reduction in lymphoid progenitors and differential expression of numerous lineage-associated genes, including significant elevation of Nfkbiz. Single-cell ATAC-sequencing analysis of DKO cells revealed increased chromatin accessibility in a set of myeloid lineage genes with increased accessibility of regions enriched in nuclear factor κB (NF-κB), GATA, and IRF family-binding motifs. To demonstrate that Nfkbiz is a direct target of Reg1/3, the authors utilized luciferase reporter constructs harboring the 3′ UTR of Nfkbiz and showed that Reg1/3 inhibited reporter activity using nuclease-dead Reg1/3 mutants. The authors showed that Reg1/3 restrict the expression of Nfkbiz by directly binding to SL structures within Nfkbiz’s 3′ UTR. Overexpression of Nfkbiz in mouse primary MPPs promoted myeloid lineage differentiation to a degree similar to CCAAT-enhancer-binding proteins, C/EBPα and C/EBPβ. The authors then showed that antisense oligonucleotides (ASO) that block the binding of Reg1/3 to Nfkbiz is sufficient to promote myeloid maturation in vitro. Finally, the authors demonstrated that the lineage bias in DKO mice is mediated, at least in part, through Nfkbiz, as crossing DKO mice onto the Nfkbiz null background resulted in improved HSPC numbers and fully restored the number of mature B and myeloid cells.
Reg1/3 degrade Nfkbiz mRNA and mediate HSPC lineage choice. Reg1/3 normally ensure that Nfkbiz is degraded, thereby preserving balanced lymphomyeloid output. However, in the absence of Reg1/3, HSC/MPP become strongly myeloid skewed. Figure adapted from the article by Uehata et al that begins on page 243.
Reg1/3 degrade Nfkbiz mRNA and mediate HSPC lineage choice. Reg1/3 normally ensure that Nfkbiz is degraded, thereby preserving balanced lymphomyeloid output. However, in the absence of Reg1/3, HSC/MPP become strongly myeloid skewed. Figure adapted from the article by Uehata et al that begins on page 243.
Although these studies provide rigorous support for a novel posttranscriptional mechanism that regulates HSPC fate and identifies a potential target for therapies to regulate hematopoietic output, several important questions remain. Although Reg1/3 clearly control hematopoietic lineage fate in immature precursors, it remains unclear if Reg1/3 act at the level of the long-term (LT)-HSC, short-term (ST)-HSC, specific MPPs, or multiple HSPC populations. This is an important issue since any treatment strategies designed to modulate the Reg1/3-Nfkbiz axis may induce more lasting or short-term phenotypes depending on in which HSPC population this pathway is active. Given that highly purified DKO LT-HSCs do not exhibit defects in engraftment, this suggests either that Reg1/3 alter HSC fates without altering self-renewal or that they alter lineage fate in more downstream progenitors. In addition, it remains unclear if Reg1/3 regulate lymphoid-myeloid fate decisions in other contexts associated with altered lymphomyeloid output including infection and aging. Finally, although the ASO studies provide support for a potential strategy to modulate hematopoietic output, they were performed under nonphysiologic conditions in which oligonucleotide was transfected directly into HSPCs followed by an in vitro readout. Whether or not oligonucleotide strategies can be utilized to effectively target Nfkbiz in vivo is presently unclear, but given the challenges of therapeutic targeting of the bone marrow using oligonucleotide strategies,7 this may prove to be quite challenging.
Taken together, these impressive studies provide important new insights into the importance of posttranscriptional mechanisms in lineage fate decisions in the most primitive hematopoietic cells. Given the importance of this pathway in regulating lineage fates during steady-state and posttransplantation hematopoiesis, further investigation is warranted on the role of Reg1/3 in HSPCs in the context of aging as well as acute and chronic inflammatory stimuli including infection and metabolic stressors such as hyperglycemia and hyperlipidemia.8,9 Indeed, it would be important to ascertain whether the Reg1/3 DKO phenotypes themselves depend on the cell-intrinsic induction of local or systemic inflammation, given the critical role of Nfkbiz in regulating NF-kB, a well-described mediator of inflammation.10 Finally, given the large body of literature demonstrating that driver mutations in myeloid neoplasms frequently induce myeloid-biased maturation that is important to promote the selection of mutant clones, it also would be of interest to determine whether the Reg1/3-Nfkbiz axis plays a role in this process.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal