In this issue of Blood, Asquith et al reveal that only a marginal fraction of extramedullary megakaryocytes (MKs) are found within the lung and the spleen in mice, suggesting that the bone marrow (BM) is the primary site for platelet production.1
MKs are large, polyploid cells, primarily responsible for platelet production,2 a process that occurs first in the yolk sac during embryogenesis, progressing to the fetal liver and ultimately to the adult BM. Despite MKs being observed in multiple tissues, the role of the contribution of the extramedullary MKs to circulating platelet mass has only recently been raised, with data suggesting a significant contribution by lung MKs.3
A study published in 2017 challenged the belief that platelets are produced primarily in the BM sinusoids. Using intravital microscopy and reporter mice to explore platelets and MKs, the authors found via direct visualization that MKs extended fine proplatelet structures within the lung. Based on imaging calculations, they estimated that lung MKs could contribute up to 50% of the platelet production in mice.3 Subsequent publications reported the presence of intravascular MKs dedicated to platelet production in addition to the resident MK populations with immunoregulatory functions in lung parenchyma.4
In this work, Asquith and colleagues reassert the BM as the primary site for thrombopoiesis, presenting compelling evidence of the critical function of the BM for platelet production. They demonstrate in mice that (1) BM exhibits significantly higher counts of MKs and progenitor cells compared with spleens or perfused lungs; (2) fetal liver and BM have the highest concentration of MKs, with fewer in the spleen and only a nominal presence in the lung identified by in situ fluorescent labeling of cryo-sections using antibodies targeting canonical MK markers, such as CD41, glycoprotein IX (GPIX), and platelet factor 4 (PF4); (3) confirmation by 2-photon intravital and light-sheet microscopy that there are greater number of MKs in the fetal liver and BM than in the spleen or lung; (4) MKs cultured ex vivo from the BM display a superior proplatelet-formation capacity in static or microfluidic assays, compared with MKs from other sources; and (5) only a limited number of cells from murine and human lung single-cell RNA-sequencing (scRNA-seq) data sets express MK markers.
The strength of this study lies in its comprehensive approach, including whole-mount light sheet and quantitative histological imaging, flow cytometry, intravital imaging, and examination of scRNA-seq databases. These methods were used to distinguish the relative abundance of MKs in various organs and to estimate their potential role in maintaining the platelet population under steady-state conditions. Based on cell frequencies, immunophenotypes, and functional characteristics of MKs from the different tissues, the study concluded that lung and spleen MKs may make only a minimal contribution to physiological platelet generation compared with their better established counterparts derived from the BM and fetal liver during development.
The observed low frequency of MKs in the spleen is in line with the existing understanding of splenic MKs serving as a significant source of platelets in inflammatory conditions, during immune responses following infections or in cases of failed BM hematopoiesis.5 What adds intrigue to this investigation is the sporadic detection and characterization of lung MKs using both conventional and cutting-edge imaging techniques, which have generated contradictory results in the literature. Previous studies have identified lung MKs using antibodies such as anti-CD42c antibody (GPIBB)4 or anti CD41/CD42d (GPV),6 whereas the current research used CD41, GPIX, and PF4 staining, which detected only a small number of MKs. Furthermore, direct evidence of physiological platelet release in the lung was reported based on in vivo MK tracking in PF4-Cre x mT/mG reporter mice,3 but physiologic platelet release was not consistently observed in vWF-eGFP reporter mice used in this study. Hence, lung MKs appear to be a distinct group from those in the adult BM and fetal liver. Recent studies have characterized lung MKs as inflammatory cells.3 Platelets are also considered inflammatory cells, and there is a well-known association between inflammation in the lung and platelets.7 Emerging evidence, from scRNA-seq studies, is revealing distinct MK subpopulations,8 which may share markers despite being heterogeneous in terms of morphology, function, and ontogeny.
In this intricate landscape, it is valuable to draw insights from clinical observations. Through the analysis of countless human peripheral blood smears, we know that under normal physiological conditions, circulating MKs are a rarity, except in pathological conditions, such as myeloproliferative neoplasms. Can MKs potentially originate directly from the lung in myeloproliferative neoplasms?9 Clinical experience emphasizes that hematological diseases typically originate in the BM, with alterations there leading to changes in peripheral blood cell counts and function.
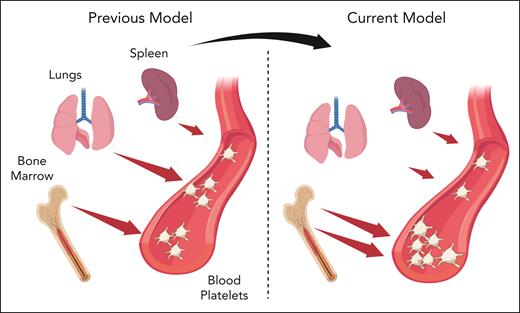
In this context, the study conducted by Asquith and colleagues reasserts the central role of BM MKs in regulating the circulating platelet mass (see figure). With the rapid advancements in technology, numerous emerging tools are providing new insights into these intricate mechanisms. For a proper interpretation of this complex data, it will be necessary to continuously reference and confirm using clinical observations. This will ensure a direct connection to human physiology by considering the central functions of individual organs, including the BM.
Extramedullary megakaryocytes and contribution to physiological platelet production. Previous studies have reported that bone marrow and lung megakaryocytes equally contribute to platelet production in mice. The current model, based on the relative megakaryocyte abundance, phenotype, and ex vivo capacity of proplatelet formation, suggests that bone marrow is the primary site of thrombopoiesis, whereas extramedullary megakaryocytes in the lung and spleen are marginally involved in the maintenance of circulating platelet mass. Created with BioRender.com.
Extramedullary megakaryocytes and contribution to physiological platelet production. Previous studies have reported that bone marrow and lung megakaryocytes equally contribute to platelet production in mice. The current model, based on the relative megakaryocyte abundance, phenotype, and ex vivo capacity of proplatelet formation, suggests that bone marrow is the primary site of thrombopoiesis, whereas extramedullary megakaryocytes in the lung and spleen are marginally involved in the maintenance of circulating platelet mass. Created with BioRender.com.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal