In this issue of Blood, Ningtyas et al1 present a novel mechanism for red blood cell (RBC) clearance involving binding of platelets to aged, senescent RBCs, enhancing their removal by erythrophagocytosis in the spleen. This highlights an important physiological function of RBC and platelet aggregates, termed platelet RBC complexes (P-RBCs), which is likely to play a critical role also in the pathology of diseases such as sickle cell disease, malaria, and immune thrombocytopenia.2,3
Abundant RBCs in the blood cause margination of platelets in vessels, through the rheological properties of particle distribution in laminar flow conditions. This is critical for normal platelet function as sentinels of endothelial damage and vessel integrity.4 RBCs and platelets are able to interact directly, also, through mechanisms that include platelet αIIbβ3 and RBC ICAM4.5 RBCs can contribute then to platelet activation, principally through release of adenosine 5′-diphosphate, under certain circumstances.4 This commentary focuses on a functionally specific interaction between platelets and senescent RBCs, mediating their clearance from the blood in healthy, and possibly disease, conditions.
Clearance of senescent RBCs is a complex process that can be mediated by a variety of mechanisms and is essential not only for recycling of their components such as iron but also to minimize procoagulant activity and thrombotic complication associated with enhanced surface exposure of phosphatidylserine (PS) on senescent RBCs.6 RBC clearance occurs primarily in the spleen, where mechanical filtration of cells with a compromised structure due to age and damage occurs in the red pulp.6 Taking advantage of their decreased deformability, RBCs are then lysed and cleared by splenic macrophages and by the reticuloendothelial system in the process of erythrophagocytosis.6 Membrane changes that signal senescence include increased surface PS expression, downregulation of the CD47 self-antigen, and opsonization.7
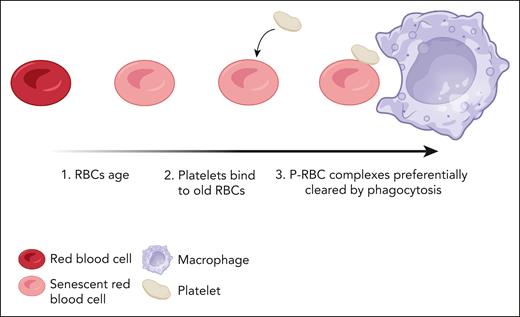
The work by Ningtyas et al advances our understanding of canonical erythrophagocytosis. The authors highlight that platelets preferentially bind to aged RBCs using a pulse-chase labeling method, tagging RBCs long term and costaining for CD41. This, combined with imaging flow cytometry, confirmed the interaction, and the authors showed the proportion of P-RBCs was 2 to 10 times greater in old vs younger RBCs. The P-RBCs are rapidly cleared by the mononuclear phagocytic system, as summarized in the figure. Mice treated with clodronate-liposomes to deplete macrophages showed decreased clearance of P-RBC aggregates, highlighting the primary role played by these cells in the clearance mechanism. When P-RBCs, RBCs, and platelets were labeled ex vivo and transfused into recipient mice, costained P-RBCs were cleared 3 times faster than platelets and 7 times faster than RBCs that were not bound by platelets. This, in conjunction with the findings of preferential binding to senescent-aged RBCs, suggests synergy between the 2 cell types when complexed together to mediate clearance of senescent RBCs.
Formation of P-RBCs leads to increased clearance of senescent RBCs. As red cells become senescent, a proportion of them are bound to platelets in circulation, forming P-RBCs. These complexes are cleared in the spleen more rapidly than unbound RBCs. Created with BioRender.com.
Formation of P-RBCs leads to increased clearance of senescent RBCs. As red cells become senescent, a proportion of them are bound to platelets in circulation, forming P-RBCs. These complexes are cleared in the spleen more rapidly than unbound RBCs. Created with BioRender.com.
It was also clear that the spleen is the primary site of clearance of P-RBCs, since elevated levels of complexes were observed in the bloodstream of splenectomized mice. This would explain the rarity of these complexes in the systematic vasculature in normal animals. Importantly, this finding translates to humans since it was shown that P-RBCs were elevated substantially 60 days postsplenectomy in human patients.
This fascinating novel discovery opens up numerous avenues for future research to examine the mechanistic nature of this enhanced clearance by binding to platelets. It will be important to determine whether platelet interaction merely enhances known mechanisms of RBC clearance, for example, by affecting RBC entrapment in splenic red pulp cords or increasing opsonization by increased presentation of autorecognition signals including PS exposure. It could, however, mediate a novel clearance mechanism in the spleen, and the authors speculate a role for platelet-derived thrombospondin bridging oxidized CD47 on the surface of senescent RBCs to the phagocytic SIRP-α receptor.8
Although the authors write this in the context of RBC clearance, could it also be possible that this process is involved in platelet clearance as well, due to the stoichiometry of the system being biased greatly in favor of RBCs? RBCs exceed platelets in both number and longevity. Does this mean, therefore, that many platelets are also cleared with an associated RBC by this mechanism? It is also interesting to speculate whether all platelets are able to interact with senescent RBCs or whether it is only a subset, for example, platelets that are also undergoing senescence. This would make sense for biology, allowing clearance of both senescent cells in a single process.
The authors also show the potential clinical importance of the process. Patients with immune thrombocytopenia (ITP) had markedly fewer P-RBCs and greater numbers of aged RBCs expressing increased levels of PS and FasR. Platelet-dependent clearance of PS-exposing RBCs in particular may be important, therefore, in minimizing intravascular coagulation but may underlie mechanisms of enhanced paradoxical thrombosis in ITP and other states of marked thrombcytopenia.9
Overall, this elegant study provides foundational work for understanding additional complexities in RBC homeostasis, and possibly platelet homeostasis also, through direct interaction between these 2 cell types. The work opens the door for further research to fully understand its mechanism and clinical implications in health and disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal