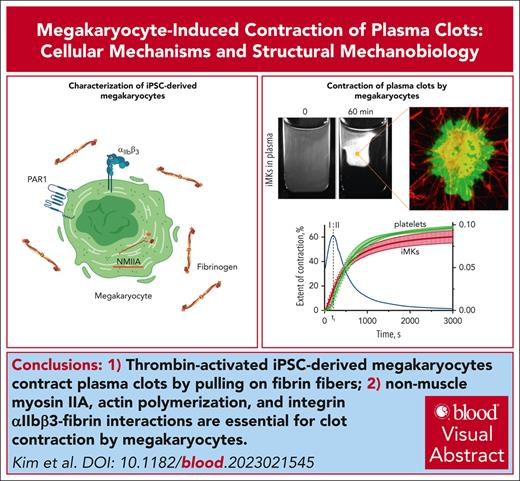
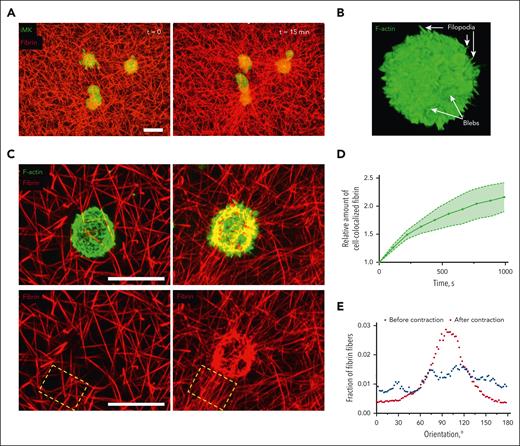
Thrombin-activated iMKs contract plasma clots by pulling on fibrin fibers with plasma membrane protrusions.
Nonmuscle myosin IIA, actin polymerization, and integrin αIIbβ3–fibrin interactions are essential for clot contraction by MKs.
Visual Abstract
Nonmuscle cell contractility is an essential feature underlying diverse cellular processes such as motility, morphogenesis, division and genome replication, intracellular transport, and secretion. Blood clot contraction is a well-studied process driven by contracting platelets. Megakaryocytes (MKs), which are the precursors to platelets, can be found in bone marrow and lungs. Although they express many of the same proteins and structures found in platelets, little is known about their ability to engage with extracellular proteins such as fibrin and contract. Here, we have measured the ability of MKs to compress plasma clots. Megakaryocytes derived from human induced pluripotent stem cells (iPSCs) were suspended in human platelet-free blood plasma and stimulated with thrombin. Using real-time macroscale optical tracking, confocal microscopy, and biomechanical measurements, we found that activated iPSC-derived MKs (iMKs) caused macroscopic volumetric clot shrinkage, as well as densification and stiffening of the fibrin network via fibrin-attached plasma membrane protrusions undergoing extension-retraction cycles that cause shortening and bending of fibrin fibers. Contraction induced by iMKs involved 2 kinetic phases with distinct rates and durations. It was suppressed by inhibitors of nonmuscle myosin IIA, actin polymerization, and integrin αIIbβ3–fibrin interactions, indicating that the molecular mechanisms of iMK contractility were similar or identical to those in activated platelets. Our findings provide new insights into MK biomechanics and suggest that iMKs can be used as a model system to study platelet contractility. Physiologically, the ability of MKs to contract plasma clots may play a role in the mechanical remodeling of intravascular blood clots and thrombi.
Introduction
Many types of nonmuscle cells generate contractile stress as an essential mechanism of diverse physiological processes, including cell spreading, motility, morphogenesis, division, intracellular transport, and secretion.1-14 Within a blood clot or thrombus, contracting platelets attach to fibrin and drive compaction and shrinkage of the entire clot both in vitro and in vivo.11-14 Importantly, defects in nonmuscle cell contractility can lead to various pathologies15,16; therefore, the ability of nonmuscle cells to contract is an important (patho)physiological mechanism and a potential therapeutic target.
Cellular contractility is driven by actin filaments interacting with myosin molecular motors, which undergo cyclic conformational transitions pulling on actin using energy from adenosine triphosphate.15,17 Nonmuscle myosin II (NMII) is the primary isoform responsible for the generation of contractile force in megakaryocytes (MKs) and platelets.18-24 Actomyosin contractility plays a critical role in thrombopoiesis, that is, the production of platelets by MKs.25-27 MKs are polyploid cells, mainly residing in bone marrow, in which they extend large protrusions (proplatelets) into the vascular lumen and release platelets into the blood flow.28-30 NMII contractility was shown to be essential for MK migration within the bone marrow as well as to avoid premature proplatelet formation25,31 and allow branching of proplatelets.25-27
Recent studies revealed the presence of MKs in lung and brain tissues and in thrombi obtained from deceased patients with COVID-19.32-34 MK counts in blood are increased in patients with severe COVID-19, including those with multiorgan injury and thrombosis,35 suggesting that MKs in blood circulation can contribute to hemostasis and thrombosis via unknown mechanisms. It is conceivable that circulating MKs can act similar to platelets, their offspring cells, which have multiple functions, including the ability to contract (retract) blood clots and thrombi. Platelet-driven clot contraction occurring in vitro and in vivo is of substantial pathophysiological importance.36-43
Despite the established and hypothetical pathophysiological importance of normal and reduced actomyosin contractility of MKs, whether MKs can cause clot contraction is unknown. Here, we studied MKs derived from human induced pluripotent stem cells (iPSC) and showed directly the ability of thrombin-activated MKs to cause contraction of fibrin clots in vitro. By applying a combination of real-time macroscale optical tracking, high-resolution confocal microscopy, and biomechanical measurements, we studied the contractile cellular mechanisms and mechanotransduction pathways in iPSC-derived MKs (iMKs) in comparison with platelets. Our findings demonstrate a new potential mechanobiological role of MKs in hemostasis and thrombosis and justify the use of iMKs as model cells for studying platelet contractility.
Materials and methods
Differentiation of iPSCs into HPCs and iMKs
The differentiation of iPSC into hematopoietic progenitor cells (HPCs) followed by expansion to iMKs was performed as previously described44-46 with modifications. Briefly, day-8 HPCs were harvested from the supernatant of a 2D differentiation culture and cryopreserved. For iMK generation, the cryopreserved HPCs were thawed and cultured in StemSpan SFEM II (Stemcell Technologies) medium with thrombopoietin (50 ng/mL; R&D) and stem cell factor (25 ng/mL; R&D) for 4 to 5 days. Fresh medium was added directly to the wells every 2 days.
Surface marker characterization of unstimulated and thrombin-stimulated iMKs
The iMKs were resuspended in Tyrode’s buffer containing 0.1% bovine serum albumin and activated with human thrombin (1 U/mL final; MilliporeSigma, catalog number T4393). Flow cytometry was performed using a CytoflexLX (BD Biosciences), and data were analyzed using the FlowJo10 software (Tree Star Inc) (Figure 1A–F).
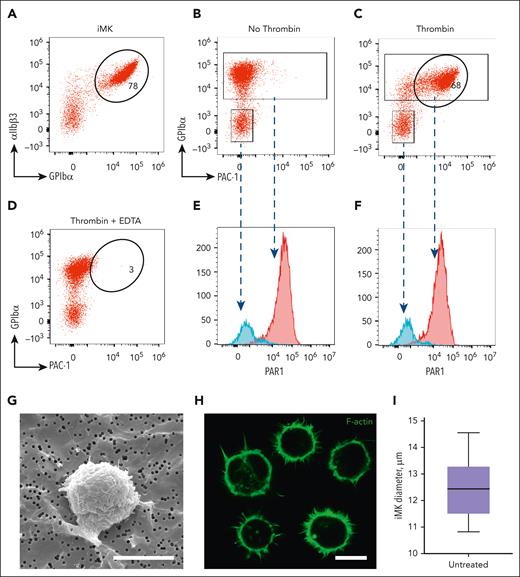
Characterization of iMKs using flow cytometry and light and electron microscopy. (A) A representative flow cytometry dot plot of unstimulated iMKs showing a high expression level of glycoprotein GPIbα. (B-C) Representative flow cytometry dot plots showing no PAC1 binding to unstimulated iMKs (B) and pronounced binding of PAC1 (expression of activated αIIbβ3) to thrombin-stimulated iMKs. (D) In the presence of 10 mM EDTA (negative control), thrombin-stimulated iMKs show no PAC1 binding. (E-F) Representative histograms derived from the flow cytometry plots B and C, respectively, showing a high level of PAR-1 expression in both unstimulated and thrombin-stimulated iMKs (red peaks) and a low level of PAR-1 in the contaminating non-iMK cells not expressing GPIbα (negative control, blue peaks). (G-H) Representative scanning electron microscopy (G) and confocal light microscopy (H) images of individual unstimulated iMKs. Scale bars, 12 μm. (I) Diameters of unstimulated iMKs assessed using the confocal microscopy images shown in H (50 cells analyzed; results are presented as the median and interquartile range (IQR) between the 25th and 75th percentiles as well as 5th and 95th percentiles).
Characterization of iMKs using flow cytometry and light and electron microscopy. (A) A representative flow cytometry dot plot of unstimulated iMKs showing a high expression level of glycoprotein GPIbα. (B-C) Representative flow cytometry dot plots showing no PAC1 binding to unstimulated iMKs (B) and pronounced binding of PAC1 (expression of activated αIIbβ3) to thrombin-stimulated iMKs. (D) In the presence of 10 mM EDTA (negative control), thrombin-stimulated iMKs show no PAC1 binding. (E-F) Representative histograms derived from the flow cytometry plots B and C, respectively, showing a high level of PAR-1 expression in both unstimulated and thrombin-stimulated iMKs (red peaks) and a low level of PAR-1 in the contaminating non-iMK cells not expressing GPIbα (negative control, blue peaks). (G-H) Representative scanning electron microscopy (G) and confocal light microscopy (H) images of individual unstimulated iMKs. Scale bars, 12 μm. (I) Diameters of unstimulated iMKs assessed using the confocal microscopy images shown in H (50 cells analyzed; results are presented as the median and interquartile range (IQR) between the 25th and 75th percentiles as well as 5th and 95th percentiles).
Scanning electron microscopy of unstimulated and thrombin-stimulated iMKs
Scanning electron micrographs of iMKs prepared as described in the supplemental Methods (available on the Blood website), were taken with a Quanta 250FEG (FEI, Hillsboro, Oregon) microscope (Figure 1G).
Blood collection and fractionation
Blood was drawn from healthy volunteers not taking antiplatelet medications for at least 14 days. Informed consent was obtained in accordance with a protocol approved by the University of Pennsylvania Institutional Review Board. Platelet-rich plasma (PRP) and platelet-free plasma were obtained by centrifugation of whole citrated blood.
Clot contraction assay
Macroscopic clot size changes were tracked optically as described12 (details in supplemental Methods).
Measurement of the contractile force generated by activated iMKs and platelets in plasma clots
The contractile force induced by fibrin-attached activated iMKs was measured with a rheometer (ARG2; TA Instruments).
Formation of iMK-containing plasma clots and imaging with a confocal microscope
Clot formation and activation of iMKs were induced by human thrombin (1 U/mL final; MilliporeSigma, catalog no. T4393) and CaCl2 (3 mM final) added to the iMK-containing plasma samples. Fluorescently labeled live iMKs and fibrin were imaged in a Zeiss LSM880 laser scanning confocal microscope.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 9.
For details of methods described above, see supplemental Methods.
Results
Surface marker characterization and morphology of iMKs
To confirm that iMKs used in this study express the platelet integrin αIIbβ3 and are sensitive to activation with thrombin, we performed flow cytometry of unstimulated and thrombin-stimulated iMKs treated with fluorescently labeled antibodies to assess the expression of the αIIb subunit (CD41), the β3 subunit (CD61), GPIbα (CD42b), and the PAR1 receptor. The major fraction of unstimulated cells expressed αIIbβ3 and GPIbα (∼78%) (Figure 1A) but did not bind PAC-1, a monoclonal antibody specific for the activated conformation of αIIbβ347 (Figure 1B). Stimulation of iMKs with thrombin resulted in PAC-1 binding (∼68%), indicating activation of αIIbβ3 (Figure 1C). When the αIIbβ3 integrin on iMKs was disrupted by treatment with ethylenediamine tetraacetic acid (EDTA) (negative control), the iMKs showed no PAC-1 binding. Both unstimulated and thrombin-stimulated population of iMKs expressed protease-activated receptor PAR1 on the cell surface (Figure 1E-F), with the expression level being fourfold higher than that of the cells not expressing GPIbα. Furthermore, scanning electron microscopy and confocal microscopy both revealed that iMKs had a median diameter of 11.4 μm (compared with platelet diameter of 3-4 μm) and generated multiple filopodia on the cell surface (Figure 1G-I). Thus, similar to platelets, iMKs expressed αIIbβ3 integrins and PAR1 receptors on their surface and were activated by thrombin, leading to the activation of αIIbβ3.
Contractility of thrombin-stimulated iMKs
To determine whether thrombin-stimulated iMKs can contract fibrin clots, our original clot contraction assay based on the optical tracking of clot size change over time was used, showing that thrombin-stimulated iMKs caused shrinkage of plasma clots (Figure 2A). The contraction rate curves of iMK-containing clots revealed 2 kinetic phases (linear and exponential phases), determined by the local extremum of the first derivative (Figure 2B). Both the images and clot contraction rate curves determined for iMKs were similar to those observed with activated platelets.12
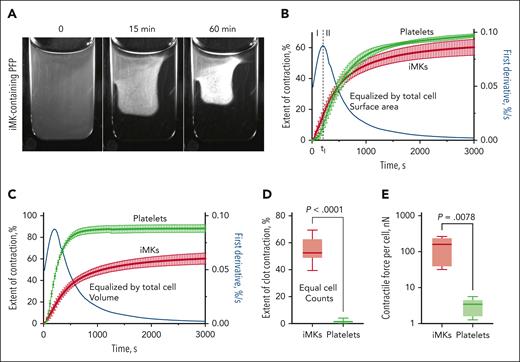
The clot contraction assay showing iMK-driven shrinkage of macroscopic plasma clots and comparative iMK and platelet contractility. (A) Representative snapshots of an iMK-containing plasma clot before contraction at zero time point as well as at 15 and 60 minutes after the onset of contraction (see supplemental Video 1). (B) Averaged contraction kinetic curves for the iMK-containing clots (red) and PRP clots (green) equalized by the cell surface area (M ± SD, n = 5). The clot contraction driven by iMKs is segregated into phases 1 and 2 defined by the local extremum of the first derivative (blue line). (C) Averaged contraction kinetic curves for the iMK-containing clots (red) and PRP clots (green) equalized by the total cell volume (M ± SD; n = 5), which reveal superb biomechanical efficacy of platelets compared with iMKs. The cell counts in plasma (B-C) were adjusted such that iMKs and platelets before clot formation and contraction (100 μL initial plasma or clot volume) had about the same total cell surface area (∼250 mm2) (B) or volume (∼0.52 mm3) (C). Equalization by the total cell surface area and volume are described in “Clot contraction assay.” (D-E) Plots showing the final extents of contraction (D) in the iMK-containing plasma clots and PRP clots that were equalized by the cell counts, and comparative contractility of individual iMKs vs single platelets (E). M, mean; SD, standard deviation.
The clot contraction assay showing iMK-driven shrinkage of macroscopic plasma clots and comparative iMK and platelet contractility. (A) Representative snapshots of an iMK-containing plasma clot before contraction at zero time point as well as at 15 and 60 minutes after the onset of contraction (see supplemental Video 1). (B) Averaged contraction kinetic curves for the iMK-containing clots (red) and PRP clots (green) equalized by the cell surface area (M ± SD, n = 5). The clot contraction driven by iMKs is segregated into phases 1 and 2 defined by the local extremum of the first derivative (blue line). (C) Averaged contraction kinetic curves for the iMK-containing clots (red) and PRP clots (green) equalized by the total cell volume (M ± SD; n = 5), which reveal superb biomechanical efficacy of platelets compared with iMKs. The cell counts in plasma (B-C) were adjusted such that iMKs and platelets before clot formation and contraction (100 μL initial plasma or clot volume) had about the same total cell surface area (∼250 mm2) (B) or volume (∼0.52 mm3) (C). Equalization by the total cell surface area and volume are described in “Clot contraction assay.” (D-E) Plots showing the final extents of contraction (D) in the iMK-containing plasma clots and PRP clots that were equalized by the cell counts, and comparative contractility of individual iMKs vs single platelets (E). M, mean; SD, standard deviation.
One characteristic of cell contractility that determines the rate and extent of clot contraction is the contractile stress, defined as force/cell surface area. To compare the relative contractile potential of iMKs and platelets, we tracked in parallel the contraction of iMK-containing plasma clots and PRP clots prepared from cell-enriched plasma samples equalized by the total cell surface area (∼250 mm2 per 100 μL of initial clot volume). Remarkably, the kinetic clot contraction curves for the 2 cell types revealed similar behavior under the conditions applied, and the final extents of clot contraction were statistically indistinguishable (P = .06) (Figure 2B).
Another approach to evaluate the relative contractile mechanical effectiveness of iMKs and platelets was to compare contraction of iMK-containing plasma clots and PRP clots prepared from cell-enriched plasma samples equalized by the total cell volume (∼0.52 mm3 per 100 μL of the initial plasma volume). To reach equal total cell volumes, the cell counts were adjusted to 5000 per μL for iMKs and 58 000 per μL for platelets, corresponding to the difference in the average individual cell volume. The analysis of the clot contraction kinetic curves revealed that the mean extent of contraction (at 60 minutes of contraction) and the average velocity of PRP clots were higher than that in iMK-containing clots by 29% and 33%, respectively (P < 10−3), implying that more platelets are required to get the same effect produced by the much larger iMKs (Figure 2C).
To compare the contractility of individual cells, we performed the clot contraction assay in iMK-containing plasma and PRP equalized by the cell counts (5000 cells per 1 μL of plasma) (Figure 2D). The average maximal extent of clot contraction induced by the same number of iMKs was about 30-times higher than that induced by platelets (P < 10−3), suggesting that individual iMKs outperform platelets when pulling on fibrin.
To quantify directly the absolute contractile force generated by activated iMKs in the fibrin network and compare it with platelet contractility, iMK-containing clots and PRP clots were prepared as described in “Measurement of the contractile force generated by activated iMKs and platelets in plasma clots” between 2 horizontal parallel plates of a rheometer, and the normal (vertical) force generated by the clot was measured at 60 minutes of clot contraction. The average maximal bulk stress developed by the entire iMK-containing clots was 23 ± 16 Pa, and for the PRP clots, it was 18 ± 9 Pa. After normalization by the number of cells in the clots, the average maximal contractile force per individual iMK in the clot was found to be 145 ± 98 nN, which was more than 1 order of magnitude higher than the force of 3.3 ± 1.7 nN generated by single platelets in PRP clots (Figure 2E). In line with the dramatically different extents of clot contraction induced by iMK and platelets under the same cell counts, these findings confirm that individual iMKs are mechanically stronger than platelets, likely due to iMKs being bigger and containing a larger amount of force-generating actomyosin.
Thus, thrombin-stimulated iMKs are contractile cells that are able to shrink macroscopic plasma clots with biphasic kinetics, similar to the contraction of PRP clots. Remarkably, iMK-containing clots and PRP clots equalized by the total cell surface area demonstrated comparable contraction rates and extent of contraction. During contraction of clots equalized by the total cell volume, platelets revealed a higher biomechanical efficacy than iMKs, due to the much higher platelet count than that of iMKs and a higher surface/volume ratio. Meanwhile, the contractility of individual iMKs, either assessed by the ability to shrink clots or measured directly, was much stronger than that of single platelets corresponding to the larger size of individual iMKs compared than that of platelets.
Cellular mechanisms of the iMK-driven clot contraction
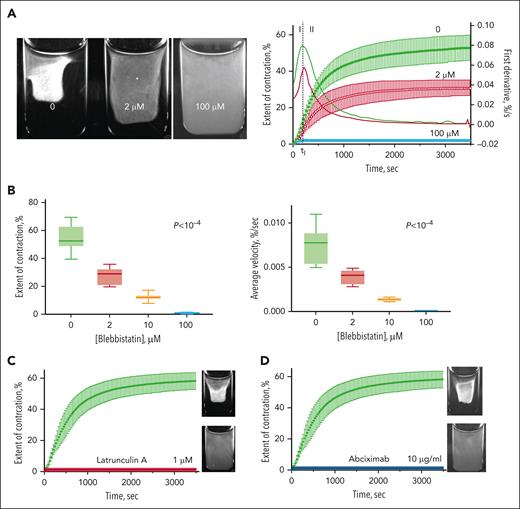
To determine whether the cellular mechanisms of iMK- and PRP-driven clot contraction are similar, specific inhibitors of cytoskeletal components and integrins were used. Blebbistatin (Sigma-Aldrich) was used to suppress myosin II activity, latrunculin A (Sigma-Aldrich) to inhibit actin polymerization, and abciximab (ReoPro; Eli Lilly) to block integrin αΙΙββ3−fibrin interactions. Pretreatment of iMKs with different concentrations of blebbistatin resulted in a significant decrease in the extent and average velocity of clot contraction in a dose-dependent manner (Figure 3; supplemental Figure 1). In the presence of small (2 μM) and intermediate (10 μM) concentrations of blebbistatin, the mean final extent and velocity of clot contraction of iMK-containing clots were reduced 1.9-fold and 5.5-fold, respectively, in comparison with untreated clots. A higher concentration of blebbistatin (100 μM) fully prevented contraction of the iMK-containing plasma clots. Pretreatment of iMKs with 1 μM latrunculin A, an inhibitor of actin polymerization, also completely prevented the contraction of plasma clots (Figure 3C). In addition, contraction of plasma clots by activated iMKs was abrogated by 10 μg/mL abciximab, the Fab of a monoclonal antibody that prevents binding of fibrin(ogen) to integrin αΙΙββ3 receptors.
Suppressive effects of specific cellular inhibitors on plasma clot contraction driven by activated iMKs. (A, left) Images of the iMK-containing macroscopic plasma clots after 90 minutes of contraction in the absence and presence of 2 and 100 μM of blebbistatin. (A, right) Averaged kinetic contraction curves of iMK-containing plasma clots in the absence (control) and presence of 2 and 100 μM blebbistatin (mean ± SD; n = 6-11). (B) The extent of contraction (left) and average contraction velocity (right) of iMK-containing plasma clots in the absence and presence of 2 μM, 10 μM, and 100 μM of blebbistatin. Results are presented as the median and IQR. Statistical significance tested using the Kruskal-Wallis test. (C-D) Complete abrogation of the iMK-induced clot contraction by 1 μM latrunculin A (C) (red curve) and 10 μg/mL abciximab (D) (blue curve). The corresponding photographic images show contracted (top) and uncontracted (bottom) clots after 2 hours of incubation in the absence (top) and presence (bottom) of the inhibitors. IQR, interquartile range; SD, standard deviation.
Suppressive effects of specific cellular inhibitors on plasma clot contraction driven by activated iMKs. (A, left) Images of the iMK-containing macroscopic plasma clots after 90 minutes of contraction in the absence and presence of 2 and 100 μM of blebbistatin. (A, right) Averaged kinetic contraction curves of iMK-containing plasma clots in the absence (control) and presence of 2 and 100 μM blebbistatin (mean ± SD; n = 6-11). (B) The extent of contraction (left) and average contraction velocity (right) of iMK-containing plasma clots in the absence and presence of 2 μM, 10 μM, and 100 μM of blebbistatin. Results are presented as the median and IQR. Statistical significance tested using the Kruskal-Wallis test. (C-D) Complete abrogation of the iMK-induced clot contraction by 1 μM latrunculin A (C) (red curve) and 10 μg/mL abciximab (D) (blue curve). The corresponding photographic images show contracted (top) and uncontracted (bottom) clots after 2 hours of incubation in the absence (top) and presence (bottom) of the inhibitors. IQR, interquartile range; SD, standard deviation.
These data show that myosin IIA, actin polymerization, and integrin-fibrin interactions are critically important for iMK contractile function, demonstrating that the cellular mechanism of iMK contractility is similar or identical to that of platelets.11
Structural changes in the fibrin network are propelled by plasma membrane protrusions of thrombin-stimulated iMKs
To analyze the structural mechanisms driving contraction of plasma clots by thrombin-activated iMKs, high-resolution confocal microscopy of individual iMKs embedded into the fibrin network within plasma clots was performed. The cells were either live or fixed along with fibrin immediately after clot formation and at various time points of contraction (Figures 4A-C and 5). The images of fixed cells showed clearly that activated iMKs formed thin filopodia and larger roundish cytoplasmic protrusions or blebs (Figure 4B) that were attached to fibrin fibers. The snapshots were complemented with time-lapse confocal microscopy of live iMKs within the fibrin network (Figures 4A and 5; supplemental Video 2). The dynamic observations revealed that, in the course of clot contraction, activated iMKs accumulated fibrin on their surface (Figure 4C). The quantitative analysis of colocalization of iMKs and fibrin at various time points showed that the amount of fibrin amassed on the surface of iMKs increased more than twofold at 15 minutes of clot contraction (Figure 4D). It is noteworthy that iMK-mediated clot contraction was followed by the radial orientation of fibrin fibers around the cells, as if they were emanating from the cell surface (Figure 4E). The actively extending and contracting blebs and filopodia attach to and pull on the fibrin fibers, causing their bending (Figure 5). Furthermore, we observed compaction of the iMK-attached fibrin fibers (supplemental Figure 2) coinciding with the contraction of the iMK cell body.
Structural bases of the interactions of thrombin-stimulated iMKs with a fibrin network during contraction of a plasma clot. (A) Representative confocal microscopy images of calcein-labeled iMKs (green) and Alexa fluor–labeled fibrin network (red) before (left) and at 15 minutes (right) of clot contraction. The images are projections of 26-μm z-stacks. Scale bar, 20 μm. See also supplemental Video 2. (B) A representative confocal microscopy image of an individual thrombin-stimulated iMK stained for F-actin within a clot fixed immediately after formation. Arrows show filopodia and blebs on the surface of the iMK. (C, upper panel) A thrombin-stimulated individual iMK embedded into the fibrin network fixed at the 0 point (left) and at 15 minutes (right) of clot contraction. (C, bottom panel) Deformation, radial orientation of fibrin fibers, and compaction of fibrin are caused by the contracting iMK shown on the upper panel (the red fluorescence channel only). Scale bars, 20 μm. (D) Temporal increase of the amount of fibrin accumulated on individual iMKs over the course of contraction, measured as a relative amount of iMK-colocalized fibrin in confocal microscopy z-stacks of clots (n = 20; mean ± 95% CI). (E) A representative diagram showing orientation of fibrin fibers in the selected field in C (dashed rectangle) in the vicinity of a contracting iMK. CI, confidence interval.
Structural bases of the interactions of thrombin-stimulated iMKs with a fibrin network during contraction of a plasma clot. (A) Representative confocal microscopy images of calcein-labeled iMKs (green) and Alexa fluor–labeled fibrin network (red) before (left) and at 15 minutes (right) of clot contraction. The images are projections of 26-μm z-stacks. Scale bar, 20 μm. See also supplemental Video 2. (B) A representative confocal microscopy image of an individual thrombin-stimulated iMK stained for F-actin within a clot fixed immediately after formation. Arrows show filopodia and blebs on the surface of the iMK. (C, upper panel) A thrombin-stimulated individual iMK embedded into the fibrin network fixed at the 0 point (left) and at 15 minutes (right) of clot contraction. (C, bottom panel) Deformation, radial orientation of fibrin fibers, and compaction of fibrin are caused by the contracting iMK shown on the upper panel (the red fluorescence channel only). Scale bars, 20 μm. (D) Temporal increase of the amount of fibrin accumulated on individual iMKs over the course of contraction, measured as a relative amount of iMK-colocalized fibrin in confocal microscopy z-stacks of clots (n = 20; mean ± 95% CI). (E) A representative diagram showing orientation of fibrin fibers in the selected field in C (dashed rectangle) in the vicinity of a contracting iMK. CI, confidence interval.
Time-lapse confocal microscopy of a live thrombin-stimulated iMK reveals bending and displacement of fibrin fibers caused by the contracting plasma membrane protrusions. (A) A representative image of a thrombin-stimulated iMK (1 of 11 individual iMKs analyzed) embedded into the fibrin network of a contracting plasma clot. Arrows indicate 2 blebs and a filopodium attached to and pulling on the fibrin fibers. Scale bars, 10 μm. (Top panel) combined fibrin and iMK images; (middle panel) iMK only; (bottom panel) fibrin network only. (B-C) Zoomed images of the contracting blebs (B) and a filopodium (C) indicated in panel A at different time points, showing retraction of the plasma membrane that induces bending and displacement of the attached fibrin fibers. White dashed lines indicate the initial profiles of the iMK emerged protrusions (middle panels) or fibrin (bottom panels). Scale bars, 2 μm. See supplemental Video 3 for the full sequence.
Time-lapse confocal microscopy of a live thrombin-stimulated iMK reveals bending and displacement of fibrin fibers caused by the contracting plasma membrane protrusions. (A) A representative image of a thrombin-stimulated iMK (1 of 11 individual iMKs analyzed) embedded into the fibrin network of a contracting plasma clot. Arrows indicate 2 blebs and a filopodium attached to and pulling on the fibrin fibers. Scale bars, 10 μm. (Top panel) combined fibrin and iMK images; (middle panel) iMK only; (bottom panel) fibrin network only. (B-C) Zoomed images of the contracting blebs (B) and a filopodium (C) indicated in panel A at different time points, showing retraction of the plasma membrane that induces bending and displacement of the attached fibrin fibers. White dashed lines indicate the initial profiles of the iMK emerged protrusions (middle panels) or fibrin (bottom panels). Scale bars, 2 μm. See supplemental Video 3 for the full sequence.
Thus, contracting iMKs form thin filopodia and larger roundish blebs, which pull on the surrounding fibrin fibers, causing densification of the fibrin network, radial orientation and bending of fibrin fibers, and accumulation of fibrin on the surface of the cells.
Kinematics of the plasma membrane protrusions in thrombin-activated iMKs
Plasma membrane protrusions of iMKs are the key cellular elements causing the remodeling of the fibrin network and shrinkage of the plasma clot. To quantitatively assess the kinematics of the plasma membrane protrusions described in the previous section, we tracked displacements of individual filopodia and blebs on the surface of thrombin-stimulated iMKs over the course of clot contraction using serial confocal microscopy images of the contracting clots (Figure 6A-B). Measurements of filopodial displacements at different time points showed that the moving cycle of an individual filopodium included extension and retraction phases, with characteristic durations and rates. The average duration of the overall filopodium extension-retraction cycle was about 210s, with the extension phase sixfold shorter than the retraction phase (supplemental Table 1). The mean extension rate of filopodia was 1.8-fold faster than its retraction rate (Figure 6C; supplemental Table 1).
Quantitative kinematics of plasma membrane protrusions in thrombin-stimulated iMKs. (A-B) Representative time-lapse confocal microscopy imaging of the extension-retraction cycle for an individual filopodium (A) and a bleb (B) on a thrombin-stimulated iMK within a plasma clot (supplemental Videos 4 and 5). Scale bars, 1 μm. White arrowheads show snapshots of the filopodium and bleb at different time points of their extension and retraction phases. (C-D) Averaged kinematic curves for filopodia (C) and blebs (D) measured as an averaged distance of the protrusion tip from the iMK surface; each curve reveals the extension and retraction phases (mean ± 95% CI; 100 filopodia and 100 blebs analyzed in 21 cells). Significance tested using the Kolmogorov-Smirnov test. In panel C, protrusion kinematics curves for the smaller (<700 nm) and larger (>700 nm) blebs are shown in blue and red, respectively. Solid lines are log-normal fits , (C, black curve) A1 = 71.24, A2 = 105.60, A3 = 3.20; (D, red curve): A1 = 95.57, A2 = 94.27, A3 = 2.30; (D, blue curve) A1 = 29.26, A2 = 70.85, A3 = 2.84. Dashed lines indicate temporal borders between the extension and retraction phases (see supplemental Table 1). Statistical significance was tested by the Kruskal-Wallis test. (E) Extension and retraction rates for individual filopodia and smaller and larger blebs determined as the averaged slopes of the kinematic curves shown in panels C-D (median and IQR). The Mann-Whitney U test. IQR, interquartile range.
Quantitative kinematics of plasma membrane protrusions in thrombin-stimulated iMKs. (A-B) Representative time-lapse confocal microscopy imaging of the extension-retraction cycle for an individual filopodium (A) and a bleb (B) on a thrombin-stimulated iMK within a plasma clot (supplemental Videos 4 and 5). Scale bars, 1 μm. White arrowheads show snapshots of the filopodium and bleb at different time points of their extension and retraction phases. (C-D) Averaged kinematic curves for filopodia (C) and blebs (D) measured as an averaged distance of the protrusion tip from the iMK surface; each curve reveals the extension and retraction phases (mean ± 95% CI; 100 filopodia and 100 blebs analyzed in 21 cells). Significance tested using the Kolmogorov-Smirnov test. In panel C, protrusion kinematics curves for the smaller (<700 nm) and larger (>700 nm) blebs are shown in blue and red, respectively. Solid lines are log-normal fits , (C, black curve) A1 = 71.24, A2 = 105.60, A3 = 3.20; (D, red curve): A1 = 95.57, A2 = 94.27, A3 = 2.30; (D, blue curve) A1 = 29.26, A2 = 70.85, A3 = 2.84. Dashed lines indicate temporal borders between the extension and retraction phases (see supplemental Table 1). Statistical significance was tested by the Kruskal-Wallis test. (E) Extension and retraction rates for individual filopodia and smaller and larger blebs determined as the averaged slopes of the kinematic curves shown in panels C-D (median and IQR). The Mann-Whitney U test. IQR, interquartile range.
Similar to filopodia, the kinematics of iMK-derived blebs was also biphasic with extension and retraction phases. However, there were 2 distinct populations of blebs that differed in size: the smaller blebs were <700 nm, and the larger blebs were >700 nm. The 2 types of blebs had different extension-retraction kinematic parameters (Figure 6C-E; supplemental Table 1). The average duration of the overall extension-retraction cycle for the smaller blebs was about 182 seconds, with the extension phase 6.9-fold shorter than the retraction phase. The corresponding mean extension rate for small blebs was 1.5-fold higher than their retraction rates. For the larger blebs, the overall extension-retraction cycle was 272 seconds (90 seconds longer than that for the smaller blebs), with the average extension phase 4.2-fold shorter than the retraction phase. The mean extension rate of the large blebs was threefold faster than their retraction rate. The extension-retraction speed of the larger blebs was similar to that of filopodia, whereas the smaller blebs revealed somewhat slower kinematics.
Thus, the (sub)micron-size filopodia and blebs on the surface of thrombin-activated iMKs undergo cyclic motion with relatively fast extension and slow retraction phases while pulling on the surrounding fibrin fibers.
Discussion
We have demonstrated a hitherto unknown ability of MKs to shrink fibrin clots. Moreover, we have deciphered cellular mechanisms of contractility of iMKs, a type of cell that has become a valuable research tool at the interface of cell biology and hematology.48-50 First, we revealed that iPSCs can be differentiated into MKs with high expression levels of glycoprotein GPIbα and αIIbβ3, and these cells are responsive to stimulation with thrombin. Second, thrombin-stimulated iMKs were able to contract plasma clots with biphasic kinetics, accompanied by densification and stiffening of the fibrin network, similar to platelet-driven contraction of clots. Third, the MK-mediated shrinkage of fibrin was achieved through the extension-retraction cycles of fibrin-attached plasma membrane protrusions, which caused shortening and bending of fibrin fibers. Fourth, the shrinkage of fibrin clots induced by iMKs was hindered by inhibitors targeting NMIIA, as well as abolished by inhibitors of actin polymerization, and αIIbβ3-fibrin interactions, similar to the contraction of fibrin clots driven by platelets. Each of these observations has potentially important pathophysiological implications, as well as remarkable underlying cellular biomechanical and structural mechanisms discussed below.
The use of iMKs as a model system to study platelet contractility
Application of iMKs to study platelet contractility has several advantages. First, iMKs can be derived from iPSC lines generated from virtually any individual.51-53 Second, the iPSCs created from patients can be applied to recapitulate ex vivo the behavior of platelets in hematopoietic diseases, such as myeloproliferative diseases, hematopoietic malignancies, or bone marrow failure.48,49 Lastly, iPSCs can be manipulated genetically using CRISPR/Cas9 or other gene-modifying and epigenetic technologies to study particular proteins and their molecular variants54,55 (see supplemental Data for additional discussion). Altogether, these unique characteristics support the use of iMKs for reproducing in cellulo-physiological processes and hematological diseases to assess pathogenic mechanisms and develop novel treatment modalities.48,56
The significance of MK contractility in thrombopoiesis and platelet disorders
It has been shown previously that the ability of MKs to form proplatelets that penetrate the endothelial layer of sinusoids and release platelets into the bloodstream involve NMIIA.24,57-59 Mutations in the MYH9 gene encoding NMIIA heavy chain60 cause disorders with macrothrombocytopenia and a propensity for bleeding.25,42,57,61 Importantly, infusion of stem cell–derived iMKs in mouse blood circulation resulted in the in vivo formation of platelets that were similar to human donor platelets in morphology, size, and functionality.49,62,63 Therefore, the contractile iMKs can be a suitable model system to study the role of NMIIA-actin machinery and the mechanisms of MYH9 gene mutations, which affect thrombopoiesis and cause multiple structural and functional defects in platelets.
Implications of MK contractility for vascular remodeling and thrombosis
The ability of MKs to contract clots may play a role in the mechanical remodeling of intravascular blood clots and thrombi. There is a growing body of evidence for circulation of MKs in the lung microvasculature associated with in situ platelet production.64-66 It has been observed that the COVID-19 virus can lead to a substantial increase of circulating severe acute respiratory syndrome coronavirus 2–containing MKs associated with increased risk of thrombosis, multiorgan injury, and mortality.35,67 At the same time, COVID-19 is characterized by reduced platelet contractility and reduced blood clot contraction.68 To compensate for the impaired platelet production and platelet dysfunction, these MKs may contribute to the contraction of intravascular clots formed in patients with COVID-19. The presence of an intravascular population of MKs in lungs, heart, and kidneys of patients with COVID-1932-34 suggests that thrombin-activated MKs, along with platelets, may promote contraction of intravascular blood clots to reinforce a hemostatic plug or affect the course of thrombosis by a number of mechanisms, such as reducing thrombus permeability and size and, hence, the extent of vessel occlusion, altering susceptibility of a thrombus to fibrinolysis, attenuating the risk of thrombotic embolization, and so on.69 Perhaps, the MK contractility can contribute to contraction of clots formed in other inflammatory disorders, such as those associated with lung cancer, in which MKs accumulate in pulmonary arteries with MKs being trapped in the lung capillaries,70 as well as with other nonhematological diseases.71
Other pathophysiological and therapeutic implications of MK contractility
In case of bone fracture and bone marrow biopsy complications, the contractility of activated MKs may help to prevent bleeding and reduce the risk of fat embolism. Moreover, the contractility of MKs can facilitate healing of fractured bones by exerting mechanical stress on the extracellular matrix (ECM) and release of growth factors, thereby accelerating tissue repair and bone remodeling.72-76
Last but not least, MK contractility is potentially relevant to therapeutic infusion of cultured MKs into blood circulation, which has been proposed and studied as a future treatment modality of diseases associated with thrombocytopenia.49 Local accumulation of the infused circulating MKs can be significant and result in a substantial amount of MKs incorporated into blood clots/thrombi, impacting intravascular blood clot formation and contraction.
Similarities in the mechanisms of MK- and platelet-mediated clot contraction
Thrombin-activated iMKs induce contraction of fibrin (Figure 2), similar to activated platelets that drive contraction of blood clots both in vitro and in vivo.11,37,39,77,78 Like platelet-mediated clot contraction, the force-dependent shrinkage of clots containing MKs is suppressed by the cellular inhibitors for NMIIA, actin polymerization, and fibrin-αIIbβ3 interactions (Figure 3). Our findings suggest that the molecular mechanisms of MK-driven clot shrinkage are similar or identical to those of platelets and involve actomyosin contractility associated with mechanotransduction to the ECM via αIIbβ3 connected to actin via talin, vinculin, and other cytosolic proteins.79,80 Similar to platelets,11 MKs use their membrane protrusions (filopodia and blebs) undergoing extension-retraction cycles to remodel the surrounding fibrin network by pulling on fibrin fibers, inducing reorientation and compaction on the cell surface, resulting in densification of the clot (Figure 4).
Differential structural biomechanics of MKs and platelets
Unlike the similar or identical molecular machinery of platelet- and MK-driven contraction of fibrin, the microscopic structural mechanisms have important distinctions. An activated platelet undergoes successive cycles of filopodia extending, attaching to a fibrin fiber and retracting, bending, and shortening the fibers (similar to pulling a rope hand-over-hand).11 Activated MKs also interact with fibrin via filopodia, but, in contrast to platelets, iMKs generate much larger membranous blebs to pull on fibrin (Figure 4). Blebbing of thrombin-activated MKs was shown earlier in isolated human MKs activated by thrombin81,82 and rat and guinea pig MKs on subendothelial ECM,83 but the spatial dynamics of the blebs on MKs had not been studied. Here, we define the quantitative structural dynamics of transient blebs formed by MKs by describing their kinematics and ability to bind and pull on fibrin fibers (Figures 4-6). Formation of blebs is a general mechanism by which various cells interact with ECM and are involved in cell migration, apoptosis, and cytokinesis.78,84-88 Our data confirm the importance of blebs in MK-fibrin mechanotransduction and contraction of ECM, emphasizing the role of blebbing in the biomechanical structural remodeling of ECM.
Activated iMKs also generate filopodia undergoing extension and retraction, which is consistent with the filopodial dynamics observed in different cell types, including HeLa, epidermal keratinocytes, and kidney COS7 cells, where the retraction rate was slower than the growth rate,89-92 as we observed for iMK filopodia. Although the detailed mechanisms of filopodium formation and retraction in iMKs is not fully clear, it is likely to be determined by the difference between the actin polymerization speed at the tip and the retrograde flow in the filopodium, as well as the rate of myosin pulling.93
Comparative strength and mechanical efficiency of clot compaction by MK and platelets
Another remarkable comparative aspect of the compaction of clots driven by platelets and MKs is the strength of traction forces exerted on fibrin with respect to the difference in the cell size. Based on scanning electron micrographs (Figure 1), the diameter of iMKs ranges from 11 to 20 μm, whereas the mean platelet diameter from the mean platelet volume measurements is 2.6 μm, in agreement with previously reported values of 2 to 5 μm.94 After normalization of the measured bulk traction forces by cell counts, the average force generated by an individual iMK (145 nN) is more than 1 order of magnitude larger than the average force of a single platelet (3.3 nN; Figure 2E), meaning that the larger iMKs are mechanically stronger than platelets. This finding is further supported by a higher extent of clot contraction induced by the same number of iMKs or platelets embedded into otherwise identical plasma clots (Figure 2D). Comparative analysis of plasma clot contraction by iMKs and platelets, when the samples are equalized by the cell surface area or volume, further reveal the critical importance of the cell-ECM interactions and the greater biomechanical efficacy of platelets (see supplemental Data for additional discussion). The latter suggests that during thrombopoiesis, the fragmentation of MKs maintains the maximal surface to volume ratio necessary to optimize platelet contractility and mechanotransduction in hemostatic clots and pathological thrombi.
Here, we used thrombin-activated iPSC-derived MKs embedded into plasma clots to study the contractility of MKs and their biomechanical interactions with fibrin. Thrombin-activated iMKs were found to compact clots by pulling on fibrin fibers with plasma membrane protrusions that undergo extension-retraction cycles. The activity of NMIIA, actin polymerization, and integrin αIIbβ3–fibrin interactions were found to be essential for clot contraction driven by iMKs, as well as by platelets. Thus, iMKs represent a model system that can be manipulated genetically to study platelet functions and disorders, despite some quantitative differences. Our findings provide novel insights not only into the mechanobiology of thrombopoiesis, but may have important pathophysiological implications related to hemostasis, thrombosis, and platelet disorders.
Acknowledgments
The authors thank Chandrasekaran Nagaswami for helping with scanning electron microscopy of cells. The authors acknowledge the CDB Microscopy Core facility at the University of Pennsylvania for microscope use. The authors acknowledge HemaCore (Moscow, Russia) for providing a Thrombodynamics Analyzer System.
The work was supported by National Institutes of Health (NIH), National Institute of Dental and Craniofacial Research grant R21DE030294 (O.V.K.), NIH, National Heart, Lung, and Blood Institute grants P01HL146373 (O.V.K., R.I.L., D.L.F., L.F.B., J.W.W.), RO1 HL148227, RO1 HL148014, and RO1 HL159256 (J.W.W.), American Society of Hematology Scholar Award (O.V.K.), and Engineering and Physical Sciences Research Council grant EP/C513037/1 to P.R. Williams (Swansea University, Wales, United Kingdom) for the TA Instruments ARG2 rheometer. Scanning electron microscopy was supported by NIH Shared Instrumentation grant S10-OD018041 (J.W.W.).
Authorship
Contribution: All authors contributed to study conceptualization; O.V.K., A.L.G., and D.L.F. were involved in data acquisition; O.V.K. conducted formal analysis; all authors were involved in investigation; O.V.K. and R.I.L. wrote the original draft; all authors reviewed and edited the manuscript; and L.F.B. and J.W.W. were involved in supervision and project administration.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Weisel, Department of Cell and Developmental Biology, University of Pennsylvania, Perelman School of Medicine, 1154 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104-6058; email: weisel@pennmedicine.upenn.edu.
References
Author notes
Original data are available upon request from authors John W. Weisel (weisel@pennmedicine.upenn.edu) and Oleg V. Kim (olegkim@pennmedicine.edu; olegkim@vt.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Quantitative kinematics of plasma membrane protrusions in thrombin-stimulated iMKs. (A-B) Representative time-lapse confocal microscopy imaging of the extension-retraction cycle for an individual filopodium (A) and a bleb (B) on a thrombin-stimulated iMK within a plasma clot (supplemental Videos 4 and 5). Scale bars, 1 μm. White arrowheads show snapshots of the filopodium and bleb at different time points of their extension and retraction phases. (C-D) Averaged kinematic curves for filopodia (C) and blebs (D) measured as an averaged distance of the protrusion tip from the iMK surface; each curve reveals the extension and retraction phases (mean ± 95% CI; 100 filopodia and 100 blebs analyzed in 21 cells). Significance tested using the Kolmogorov-Smirnov test. In panel C, protrusion kinematics curves for the smaller (<700 nm) and larger (>700 nm) blebs are shown in blue and red, respectively. Solid lines are log-normal fits (A1/t)exp[−0.5(ln(t/A2)ln(A3))2], (C, black curve) A1 = 71.24, A2 = 105.60, A3 = 3.20; (D, red curve): A1 = 95.57, A2 = 94.27, A3 = 2.30; (D, blue curve) A1 = 29.26, A2 = 70.85, A3 = 2.84. Dashed lines indicate temporal borders between the extension and retraction phases (see supplemental Table 1). Statistical significance was tested by the Kruskal-Wallis test. (E) Extension and retraction rates for individual filopodia and smaller and larger blebs determined as the averaged slopes of the kinematic curves shown in panels C-D (median and IQR). The Mann-Whitney U test. IQR, interquartile range.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/6/10.1182_blood.2023021545/1/m_blood_bld-2023-021545-gr6.jpeg?Expires=1763506041&Signature=KBigKcn30K~r3KcDcj6jRjmmBmHhq8X8l4gh2i15Z9HgE7xKmAsbF0ohwgmksaSC1UK4ofA9NBsXGVkT36xbbsbdjvALSe-vU1aQQOSWpBNtOWmAVphuE8XSso8AkbhBE9FSM6gNc5sI4kjjwmYEEqA9q0F1o-aY4cclxBw4T0Q-yBzILLkhdEZFKAIUTr8AFT-dyYRCvMOosT26NwI4nTBJx~Y7qWbtuWI6sVg0tbOmI8GSyaHj5x4r2toCUrzXKzKI9KX4JYD0O9Jc7eP~NaWrl8HYP~FNz4svL~dXxYiHaz5w9-7~fE-96oL7eQZexeb58gTGRchebJtQFTWwEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal