Intravenous injection of excess apotransferrin enhances dietary iron absorption in mice and triggers accumulation of plasma non–transferrin-bound iron. Injected fluorescent-labeled transferrin colocalizes with lamina propria macrophages, consistent with the recently proposed iron absorption checkpoint involving macrophage-mediated transferrin degradation.
TO THE EDITOR:
Transferrin maintains plasma iron in a redox inert state and delivers it to developing erythroblasts and other cells.1 It captures iron that is either recycled from erythrophagocytic macrophages or newly absorbed from duodenal enterocytes, and is not considered to have any regulatory function on iron entry into the bloodstream. Under physiological conditions, approximately two-thirds of the protein remains as iron-free apo-transferrin (apo-Tf), to prevent accumulation of redox active and potentially toxic non–transferrin-bound iron (NTBI). We and others showed that NTBI uptake by liver sinusoidal endothelial cells induces expression of bone morphogenetic protein 6 (BMP6),2,3 an activator (together with BMP2) of the iron hormone hepcidin.4 Thus, NTBI plays a role in iron sensing.
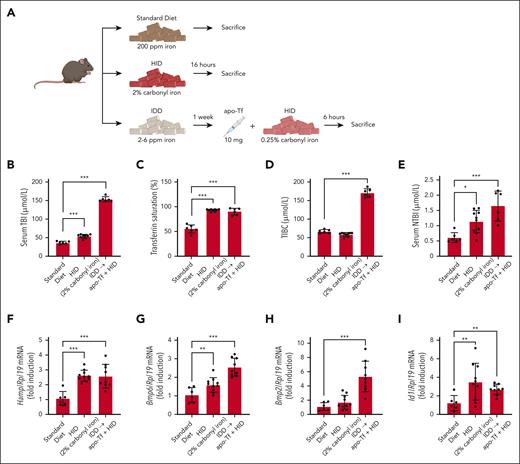
In mouse models, NTBI is typically detectable on feeding a high-iron diet (HID). Thus, in an experiment using Tfrcfl/fl (floxed transferrin receptor 1) mice,2 administration of an HID containing 2% carbonyl iron for 18 hours increased serum transferrin-bound iron (TBI) by ∼45% and transferrin saturation to ∼90%, eventually causing an ∼100% expansion of the NTBI pool compared with mice on a standard diet (Figure 1A-E). This was associated with significant induction of Hamp (hepcidin), Bmp6, and Id1 (marker of BMP signaling) mRNAs, whereas Bmp2 mRNA was not affected (Figure 1F-I). We reasoned that injection of excess apo-Tf would prevent HID-induced NTBI formation and downstream responses. To address this, mice previously fed an iron-deficient diet (IDD; for minimal NTBI baseline) were injected with 10 mg apo-Tf and switched to an HID containing 0.25% carbonyl iron for 6 hours. A lower iron content and shorter time frame compared with the HID treatment alone were chosen to allow for complete neutralization of NTBI by apo-Tf. Surprisingly, under these conditions, the animals exhibited serum TBI levels dramatically increased ∼3-fold vs mice on a standard diet, and even increased ∼2.5-fold vs mice on HID with 2% carbonyl iron for 18 hours (Figure 1B). Moreover, NTBI formation was likewise induced, rather than inhibited (Figure 1E). As expected, total iron-binding capacity (TIBC) was drastically increased following apo-Tf injection (Figure 1D), whereas transferrin saturation reached ∼90% due to HID intake. These responses were accompanied by significant induction of Hamp, Bmp6, Bmp2, and Id1 mRNAs (Figure 1F-I). Overall, the data in Figure 1 suggest that excess of exogenous apo-Tf enhances dietary iron absorption and fails to scavenge plasma iron, leading to NTBI accumulation.
Apo-Tf injection exacerbates responses to dietary iron loading and promotes NTBI formation in mice fed a high-iron diet. (A) Schematic experimental outline. Male 7- to 8-week-old Tfrcfl/fl mice (n = 6-10 per group) were fed a standard diet (200 ppm iron), iron-deficient diet (IDD; 2-6 ppm iron), or HID (2% or 0.25% carbonyl iron), as described by Charlebois et al.2 Where indicated, the mice were injected intravenously (tail vein) with 10 mg apo-Tf. At the end point, the animals were sacrificed; serum was prepared, and livers were harvested for biochemical analysis. (B) Serum TBI. (C) Transferrin saturation. (D) Total iron-binding capacity (TIBC). (E) NTBI. (F-I) Quantitative polymerase chain reaction analysis of liver Hamp, Bmp6, Bmp2, and Id1 mRNAs. Serum data (B-E) are represented as mean ± SEM; gene expression data (F-I) are represented as geometric mean ± geometric SD. Statistical differences (P < .05) were determined using 1-way analysis of variance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Apo-Tf injection exacerbates responses to dietary iron loading and promotes NTBI formation in mice fed a high-iron diet. (A) Schematic experimental outline. Male 7- to 8-week-old Tfrcfl/fl mice (n = 6-10 per group) were fed a standard diet (200 ppm iron), iron-deficient diet (IDD; 2-6 ppm iron), or HID (2% or 0.25% carbonyl iron), as described by Charlebois et al.2 Where indicated, the mice were injected intravenously (tail vein) with 10 mg apo-Tf. At the end point, the animals were sacrificed; serum was prepared, and livers were harvested for biochemical analysis. (B) Serum TBI. (C) Transferrin saturation. (D) Total iron-binding capacity (TIBC). (E) NTBI. (F-I) Quantitative polymerase chain reaction analysis of liver Hamp, Bmp6, Bmp2, and Id1 mRNAs. Serum data (B-E) are represented as mean ± SEM; gene expression data (F-I) are represented as geometric mean ± geometric SD. Statistical differences (P < .05) were determined using 1-way analysis of variance. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
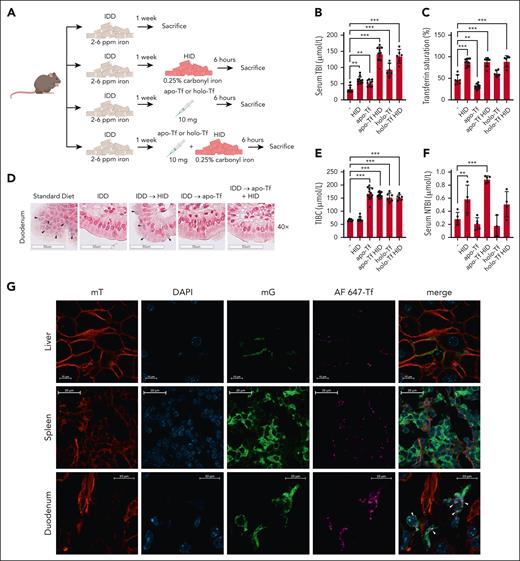
To validate these findings, wild-type mice previously fed an IDD were left untreated, injected with 10 mg apo-Tf or iron-loaded holo-transferrin (holo-Tf), or switched to HID (0.25% carbonyl iron) for 6 hours with or without apo-Tf or holo-Tf injection (Figure 2A). Expectedly, the switch to HID increased serum TBI by ∼75% (Figure 2B), transferrin saturation to ∼90% (Figure 2C), and NTBI by ∼100% (Figure 2F) but did not affect TIBC (Figure 2E). Both apo-Tf and holo-Tf further increased TBI (vs HID alone) by ∼2-fold (Figure 2B); however, only apo-Tf further increased NTBI (vs HID alone) by ∼50% (Figure 2F). IDD feeding resulted in depletion of duodenal enterocytes from histologically detectable iron, which reemerged following HID intake (Figure 2D). Importantly, apo-Tf injection prevented duodenal iron accumulation in response to HID intake (Figure 2D, right panel), indicating (together with the serum iron data) increased iron efflux. Also, apo-Tf injection without switch to HID increased TBI but did not cause any changes in NTBI or duodenal iron content, whereas, as expected, it reduced transferrin saturation and increased TIBC (Figure 2B-F). These data reinforce the interpretation that apo-Tf stimulates dietary iron absorption and does not promote mobilization of iron from stores. Injection of holo-Tf, an iron source, increased TBI (Figure 2B) but not NTBI levels (Figure 2F). Thus, it appears that excess of apo-Tf rather than holo-Tf is the major enhancer of dietary iron absorption. The stimulating effects of apo-Tf on TBI, presumably due to increased iron absorption, were also evident, yet quantitatively less pronounced, in mice switched from a standard diet to HID (supplemental Figure 1, available on the Blood website).
Excess circulating apo-Tf enhances dietary iron absorption, whereas injected fluorescent-conjugated transferrin colocalizes with lamina propria macrophages. (A) Schematic experimental outline. Male 7- to 8-week-old wild-type (C57BL/6) mice (n = 6-10 per group) were fed an IDD (2-6 ppm iron) for 1 week. Afterwards, they were switched to an HID (0.25% carbonyl iron) and/or injected intravenously (tail vein) with 10 mg apo-Tf or holo-Tf. After 6 hours, the animals were sacrificed; serum was prepared and used for analysis of iron indexes; duodenal sections were prepared and used for histologic analysis of iron. (B) Serum TBI. (C) Transferrin saturation. (D) Histologic detection of iron deposits in enterocytes by Perls staining (black arrowheads); a section from a mouse on standard diet was used as control. (E) TIBC. (F) NTBI. (G) Rosa26mT-mG/+;LysM-Cre reporter mice were generated by breeding Rosa26mT-mG/+ (see Charlebois et al2) and LysM-Cre animals. The reporter mice were injected with 10 mg apo-Tf spiked with 100 μg Alexa Fluor 647 (AF647)–conjugated transferrin. After 30 minutes, the animals were sacrificed, and tissues were collected. Frozen liver (top) and duodenal (bottom) sections were analyzed by fluorescent confocal microscopy; DAPI (4′,6-diamidino-2-phenylindole) staining was used for nuclear visualization. Signal corresponding to mT (membrane-targeted tandem dimer Tomato) is shown in red; DAPI in blue, mG (membrane-targeted enhanced green fluorescent protein) in green, and AF647-Tf in pink. Merged images are shown on the right panels; white arrowheads indicate colocalization of AF647-Tf with green macrophages. Scale bars, 20 or 10 μm. Serum data (B-E) are represented as mean ± SEM. Statistical differences (P < .05) were determined using 1-way analysis of variance, except the comparison between “-” and “apo-Tf” groups (B), which was done by the unpaired Student t test. ∗∗P < .01, ∗∗∗P < .001.
Excess circulating apo-Tf enhances dietary iron absorption, whereas injected fluorescent-conjugated transferrin colocalizes with lamina propria macrophages. (A) Schematic experimental outline. Male 7- to 8-week-old wild-type (C57BL/6) mice (n = 6-10 per group) were fed an IDD (2-6 ppm iron) for 1 week. Afterwards, they were switched to an HID (0.25% carbonyl iron) and/or injected intravenously (tail vein) with 10 mg apo-Tf or holo-Tf. After 6 hours, the animals were sacrificed; serum was prepared and used for analysis of iron indexes; duodenal sections were prepared and used for histologic analysis of iron. (B) Serum TBI. (C) Transferrin saturation. (D) Histologic detection of iron deposits in enterocytes by Perls staining (black arrowheads); a section from a mouse on standard diet was used as control. (E) TIBC. (F) NTBI. (G) Rosa26mT-mG/+;LysM-Cre reporter mice were generated by breeding Rosa26mT-mG/+ (see Charlebois et al2) and LysM-Cre animals. The reporter mice were injected with 10 mg apo-Tf spiked with 100 μg Alexa Fluor 647 (AF647)–conjugated transferrin. After 30 minutes, the animals were sacrificed, and tissues were collected. Frozen liver (top) and duodenal (bottom) sections were analyzed by fluorescent confocal microscopy; DAPI (4′,6-diamidino-2-phenylindole) staining was used for nuclear visualization. Signal corresponding to mT (membrane-targeted tandem dimer Tomato) is shown in red; DAPI in blue, mG (membrane-targeted enhanced green fluorescent protein) in green, and AF647-Tf in pink. Merged images are shown on the right panels; white arrowheads indicate colocalization of AF647-Tf with green macrophages. Scale bars, 20 or 10 μm. Serum data (B-E) are represented as mean ± SEM. Statistical differences (P < .05) were determined using 1-way analysis of variance, except the comparison between “-” and “apo-Tf” groups (B), which was done by the unpaired Student t test. ∗∗P < .01, ∗∗∗P < .001.
Sukhbaatar et al recently showed that macrophages from duodenal lamina propria negatively control dietary iron absorption by degrading transferrin via lysosomal proteases in a mammalian target of rapamycin complex 1–dependent manner.5 However, this work did not clarify whether lamina propria macrophages degrade systemic or locally produced transferrin. To address this, we used Rosa26mT-mG/+;LysM-Cre reporter mice. In this model, Cre recombination driven by the macrophage-specific LysM promoter triggers replacement of red mT (membrane-targeted tandem dimer Tomato) with green mG (membrane-targeted enhanced green fluorescent protein) in macrophages, allowing their visualization (Figure 2G). The Rosa26mT-mG/+;LysM-Cre mice were injected with 10 mg apo-Tf spiked with 100 μg fluorescent-conjugated transferrin (AF647-Tf). AF647-Tf fluorescence was detectable in liver and spleen sections (top and middle panels, respectively) around nonmacrophage red cells. By contrast, in duodenal sections (bottom panels), AF647-Tf fluorescence colocalized with green lamina propria macrophages (see also original magnification images in supplemental Figure 2). The colocalization was more prominent in crypt areas. Thus, injected AF647-Tf (and presumably apo-Tf) can enter the interstitium of the lamina propria and interact with macrophages. Together with the findings by Sukhbaatar et al,5 our data suggest that lamina propria macrophages negatively regulate dietary iron absorption by locally degrading circulating transferrin.
In conclusion, we show that injection of excess apo-Tf stimulates dietary iron absorption. Conceivably, as an iron acceptor, apo-Tf facilitates ferroportin-mediated iron efflux from duodenal enterocytes by shifting the thermodynamic equilibrium. The well-established role of the multicopper ferroxidases ceruloplasmin and hephaestin in this pathway6 is in line with the herein proposed additional requirement of apo-Tf. Immunohistochemical analysis shows that the levels of duodenal ferroportin, divalent metal transporter 1, and transferrin receptor 1 (detectable in enterocytes and lamina propria macrophages) appropriately responded to dietary iron challenges7 but were not affected by apo-Tf (supplemental Figure 3). The expression of ceruloplasmin and F4/80, a macrophage marker, remained unaffected by iron or apo-Tf, whereas hephaestin could not be detected by commercially available antibodies. These data do not provide any evidence that apo-Tf injection combined with HID intake recruits circulating ceruloplasmin in the lamina propria. Moreover, quantitative polymerase chain reaction analysis showed that hephaestin mRNA levels were rather decreased (supplemental Figure 4), presumably as a homeostatic response to limit excessive iron absorption.
It is unexpected that under our experimental conditions, excess apo-Tf could not scavenge newly absorbed iron in plasma and allowed NTBI formation. Daily injections of apo-Tf or holo-Tf over a period of 60 days were previously shown to abolish NTBI in a mouse model of β-thalassemia.8 We speculate that our model reflects short-term responses where excess apo-Tf greatly enhances dietary iron absorption but does not suffice to efficiently capture newly internalized plasma iron. Considering that effective loading of apo-Tf with iron requires multicopper ferroxidases,6 it is possible that the activity of these enzymes is limiting during acute dietary iron overload. On the other hand, chronic apo-Tf administration may allow adaptative responses that prevent NTBI formation.
In any case, circulating apo-Tf emerges as a possible new player in dietary iron absorption. It may act as a driver of iron absorption presumably by virtue of its iron acceptor function, in a process that appears to be negatively regulated by lamina propria macrophages via proteolysis.
Experimental procedures were approved by the Animal Care Committee of McGill University (protocol 4966).
Acknowledgments
S.T. was supported by a fellowship from the Canadian Institutes of Health Research. E.C. was supported by fellowships from the Natural Sciences and Engineering Research Council of Canada and subsequently the Fonds de recherche du Québec–Santé. This study was funded by a grant from the Canadian Institutes of Health Research (PJT-486651).
Authorship
Contribution: S.T., E.C., and C.F. performed research and analyzed data; K.P. designed and supervised the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kostas Pantopoulos, Lady Davis Institute for Medical Research, 3999 Cote-Ste Catherine Rd., Montreal, QC H3T 1E2; email: kostas.pantopoulos@mcgill.ca.
References
Author notes
For original data, please contact the corresponding author, Kostas Pantopoulos (kostas.pantopoulos@mcgill.ca).
There is a Blood Commentary on this article in this issue.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal