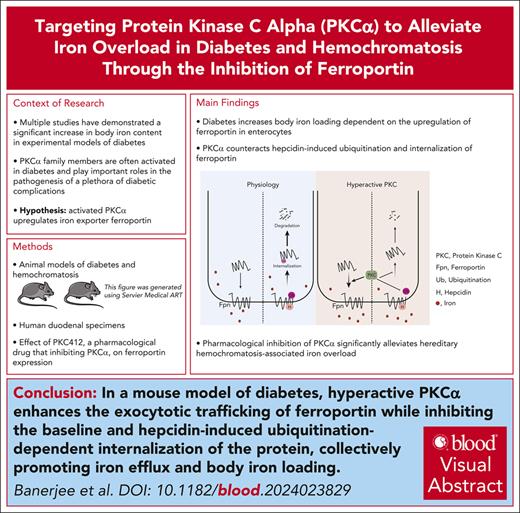
Key Points
PKCα is novel and positive regulator of Fpn by modulating the trafficking and localization of Fpn.
Inhibition of PKCα reduces body iron content in physiology, diabetes, and hereditary hemochromatosis.
Visual Abstract
Ferroportin (Fpn) is the only iron exporter, playing a crucial role in systemic iron homeostasis. Fpn is negatively regulated by its ligand hepcidin, but other potential regulators in physiological and disease conditions remain poorly understood. Diabetes is a metabolic disorder that develops body iron loading with unknown mechanisms. By using diabetic mouse models and human duodenal specimens, we demonstrated that intestinal Fpn expression was increased in diabetes in a hepcidin-independent manner. Protein kinase C (PKC) is hyperactivated in diabetes. We showed that PKCα was required to sustain baseline Fpn expression and diabetes-induced Fpn upregulation in the enterocytes and macrophages. Knockout of PKCα abolished diabetes-associated iron overload. Mechanistically, activation of PKCα increased the exocytotic trafficking of Fpn and decreased the endocytic trafficking of Fpn in the resting state. Hyperactive PKCα also suppressed hepcidin-induced ubiquitination, internalization, and degradation of Fpn. We further observed that iron loading in the enterocytes and macrophages activated PKCα, acting as a novel mechanism to enhance Fpn-dependent iron efflux. Finally, we demonstrated that the loss-of-function of PKCα and pharmacological inhibition of PKC significantly alleviated hereditary hemochromatosis-associated iron overload. Our study has highlighted, to our knowledge, for the first time, that PKCα is an important positive regulator of Fpn and a new target in the control of iron homeostasis.
Introduction
Iron is vital for life by mediating hemoglobin synthesis and fundamental cellular metabolism.1 Perturbed iron homeostasis results in iron deficiency or iron overload that in turn leads to significant health concerns.2 Systemic iron homeostasis is maintained through the tightly controlled absorption, utilization, storage, and recycling of iron.3 Ferroportin (Fpn, SLC40A1) is the only known iron exporter, playing a crucial role in regulating systemic and cellular iron homeostasis.4 Fpn is abundantly expressed in the enterocytes and macrophages that mediate dietary iron absorption and iron recycling, respectively.5 The expression and activity of Fpn is subjected to negative regulation by its ligand hepcidin6, a peptide hormone produced by the hepatocytes. Hepcidin expression is increased in response to iron overload and inflammation to suppress Fpn-mediated iron efflux.7 Hepcidin acts to occlude Fpn and induce its ubiquitination, internalization, and degradation.6,8 Disruption of the hepcidin–Fpn axis is commonly seen in hereditary hemochromatosis, an inherited disorder featured for systemic iron overload because of mutations in genes that regulate hepcidin expression or mutations in Fpn that cause resistance to hepcidin.9,10 Despite the well-characterized role of hepcidin in Fpn regulation, other potential regulators of Fpn are much less understood. The identification of molecules critical for the expression and localization of Fpn would provide novel insights into a better control of iron homeostasis and the treatment of diseases related to iron disorders.
Diabetes is a metabolic disorder that elicits a strong cross talk with the iron metabolism.11 Diabetes, characterized by elevated blood sugar level, results from insulin deficiency or insulin resistance, which underlies the pathogenesis of type 1 and type 2 diabetes, respectively.12 Elevated iron content increases the risk of diabetes, whereas diabetes further promotes body iron loading.11,13 Multiple studies, including ours, have demonstrated a significant increase in body iron content in experimental models of diabetes.14-17 It was reported that intestinal Fpn expression was increased in diabetic rats.16 Protein kinase C (PKC) family members are often activated in diabetes and play important roles in the pathogenesis of a plethora of diabetic complications.18 We previously showed that, in the intestine of diabetes, PKCα enhances the membrane expression of divalent metal transporter 1,17 a nonheme iron importer essential for dietary iron absorption.19 In this study, by using multiple diabetic mouse models, human duodenal specimens, and cultured cells, we identified that PKCα upregulated Fpn expression and was responsible for diabetic iron overload. PKCα enhanced membrane Fpn expression by decreasing the endocytic trafficking of Fpn and increasing the exocytotic trafficking of Fpn in both the resting and hepcidin-exposed conditions. The loss-of-function of PKCα significantly reduced Fpn expression and the magnitude of iron overload in diabetes and hemochromatosis. Our study defined, to our knowledge, for the first time, that PKCα is a novel regulator of Fpn and that PKCα may be targeted to control systemic iron homeostasis.
Methods
Animal models
Wild-type (WT) C57BL/6 mice, Hfe−/−, db/+, db/db, KKAy, and the a/a control mice were purchased from The Jackson Laboratory. PKCα−/− mice in C57BL/6 background were as previously described,17 and were used for crossbreeding with db/+ and Hfe−/− mice to obtain db/db;PKCα−/− and Hfe−/−;PKCα−/− mice, respectively. All mice were fed on a standard chow diet that contains 240 ppm iron. To induce type 1 diabetes, mice at the age of 6 to 8 weeks were given streptozotocin (STZ, 40 mg/kg body weight) for 5 consecutive days. Mice with fasting blood glucose levels >300 mg/dL were selected for the study. STZ-treated, db/db and KKAy mice were euthanized ∼2 months after they were hyperglycemic, unless otherwise noted. For the study with PKC412, a pharmacological drug that inhibits PKC, newly born Hfe−/− pups and their dams were fed an iron-deficient diet (TD.80396, 2-6 ppm iron) until weaning age. After weaning, mice were transferred onto a regular diet and received PKC412 (50 mg/kg per day, 200 μL dimethyl sulfoxide, PEG300, and H2O at a ratio of 5:45:50) via oral gavage for 2 weeks. At the end of PKC412 treatment, mice were euthanized, and samples were collected for the subsequent analyses.
Study approval
Animal protocols were approval by the institutional animal care and use committee of Emory University, and the human research protocol was approved by the human ethics committee of Brown University.
Additional details are provided in supplemental Materials, available on the Blood website.
Results
Diabetes increases body iron loading dependent on the upregulation of Fpn
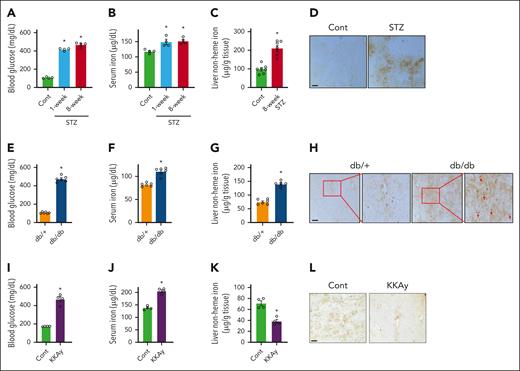
We first determined body iron status in STZ-induced type 1 diabetic mice, and type 2 diabetic db/db and KKAy mice.20 One and 8 weeks after the last injection of STZ, mice developed a prominent and comparable level of hyperglycemia (Figure 1A). Both groups displayed a significant increase in serum iron content compared twith the controls (Figure 1B). The liver is the primary site of iron deposition when iron is in excess. A significant increase was seen in hepatic nonheme iron content by biochemical assay (Figure 1C) and Perls iron staining (Figure 1D). Similarly, db/db mice, which displayed prominent hyperglycemia (Figure 1E), also manifested significant increases in serum (Figure 1F) and liver (Figure 1G) iron content compared with the db/+ controls. The db/db mice displayed iron accumulation in both the hepatocytes and Kupffer cells in the liver (Figure 1H). Diabetic KKAy mice (Figure 1I) showed a similar increase in serum iron relative to their controls (Figure 1J). However, liver iron content was significantly lower (Figure 1K-L), and the reason is unknown.
Body iron status in different diabetic mouse models. Male mice treated by (A-D) STZ, (E-H) male db/db mice, and (I-L) KKAy mice were studied in comparison with their corresponding controls (Cont). (A,E,I) Blood glucose, (B,F,J) serum iron, and (C,G,K) liver nonheme iron levels were determined. Perls iron staining was performed to show the magnitude of iron deposition in the liver of diabetic and control mice for panels D,H,L. Data are expressed as mean ± standard error of the mean (SEM; n = 4-7 mice per group). ∗P < .01 compared with the corresponding controls. Red arrows denote Kupffer cells in the liver of db/db mice. Bars, 100 μm.
Body iron status in different diabetic mouse models. Male mice treated by (A-D) STZ, (E-H) male db/db mice, and (I-L) KKAy mice were studied in comparison with their corresponding controls (Cont). (A,E,I) Blood glucose, (B,F,J) serum iron, and (C,G,K) liver nonheme iron levels were determined. Perls iron staining was performed to show the magnitude of iron deposition in the liver of diabetic and control mice for panels D,H,L. Data are expressed as mean ± standard error of the mean (SEM; n = 4-7 mice per group). ∗P < .01 compared with the corresponding controls. Red arrows denote Kupffer cells in the liver of db/db mice. Bars, 100 μm.
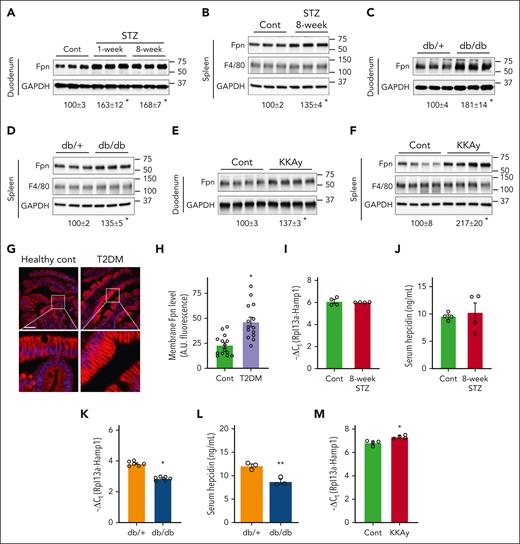
We then determined Fpn protein expression in the duodenum and spleen given their respective roles in iron absorption and iron recycling. Fpn expression was significantly increased in both tissues in STZ-treated mice (Figure 2A-B), with a more robust change in the duodenum. Increased Fpn expression were also seen in the duodenum and spleen of db/db mice (Figure 2C-D) and KKAy mice (Figure 2E-F). However, unlike STZ-treated mice and db/db mice, KKAy mice displayed a larger increase of Fpn in the spleen. The increase in splenic Fpn expression was not due to different numbers of macrophages, because the expression of F4/80, a macrophage marker, was not significantly different between the diabetic and control mice (Figure 2B,D,F). Despite the significant increases in Fpn protein levels, duodenal and splenic Fpn messenger RNA (mRNA) expression was not significantly altered except a slight increase in the db/db duodenum (supplemental Figure 1). Consistent with the increased Fpn protein expression, splenic iron content was significantly lower in all 3 diabetic models (supplemental Figure 2A). Splenic expression of ferritin heavy chain 1 (FTH1), an iron-binding protein,21 was also lower in STZ and KKAy models but not in db/db mice (supplemental Figure 2B). Similarly, a decrease of iron content was seen in the duodenum in STZ and db/db mice (supplemental Figure 2C-D).
Upregulation of Fpn expression in diabetic mice and humans. (A,C,E) Fpn protein expression in the duodenum and (B,D,F) spleen of (A-B) STZ-treated mice, (C-D) db/db mice, and (E-F) KKAy mice, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The percentages of change in Fpn expression (underneath the blots) in diabetic mice over the control are shown as mean ± SEM (n = 6 for panels A-D, and n = 4 for panels E-F). (G) Representative Fpn staining of the duodenum of type 2 diabetic (T2DM) and human patients who are nondiabetic. (H) Quantified arbitrary fluorescent intensity of membrane Fpn in T2DM and control patients. Data are mean ± SEM (n = 14 human patients). For each duodenal sample, fluorescent signal was measured in 10 representative cells by ImageJ and the average was obtained. Liver Hamp1 mRNA expression was determined by quantitative reverse transcription polymerase chain reaction in (I) STZ-treated mice, (K) db/db, and (M) KKAy mice. Serum hepcidin content was determined in (J) STZ-treated and (L) db/db mice by enzyme-linked immunosorbent assay. ∗P < .01 and ∗∗P < .05 compared with the corresponding controls. Bar, 50 μm.
Upregulation of Fpn expression in diabetic mice and humans. (A,C,E) Fpn protein expression in the duodenum and (B,D,F) spleen of (A-B) STZ-treated mice, (C-D) db/db mice, and (E-F) KKAy mice, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The percentages of change in Fpn expression (underneath the blots) in diabetic mice over the control are shown as mean ± SEM (n = 6 for panels A-D, and n = 4 for panels E-F). (G) Representative Fpn staining of the duodenum of type 2 diabetic (T2DM) and human patients who are nondiabetic. (H) Quantified arbitrary fluorescent intensity of membrane Fpn in T2DM and control patients. Data are mean ± SEM (n = 14 human patients). For each duodenal sample, fluorescent signal was measured in 10 representative cells by ImageJ and the average was obtained. Liver Hamp1 mRNA expression was determined by quantitative reverse transcription polymerase chain reaction in (I) STZ-treated mice, (K) db/db, and (M) KKAy mice. Serum hepcidin content was determined in (J) STZ-treated and (L) db/db mice by enzyme-linked immunosorbent assay. ∗P < .01 and ∗∗P < .05 compared with the corresponding controls. Bar, 50 μm.
We wondered how duodenal Fpn expression is altered in diabetic human patients. Patients with type 2 diabetes were selected given its high prevalence, and immunofluorescent staining was performed with a validated anti-human Fpn antibody (supplemental Figure 3A). The expression and localization of Fpn in the enterocytes varied among the studied individuals in both the diabetic and control groups (Figure 2G; supplemental Figure 3B). Importantly, quantification of the fluorescence intensity revealed a significantly higher membrane Fpn expression in the diabetic group (Figure 2H). Consistent with the elevated Fpn expression, intracellular iron content in the epithelia was significantly less in the diabetic group (supplemental Figure 4).
PKCα mediates baseline and diabetes-induced expression of Fpn
Hepcidin is a master regulator of Fpn, and it was reported that hepcidin deficiency is the mechanism of Fpn upregulation in STZ- and diet-induced diabetes in rats.16 However, we failed to see significant changes in hepatic hepcidin mRNA (Figure 2I) or serum hepcidin content (Figure 2J) in STZ-induced diabetic mice. Liver hepcidin mRNA (Figure 2K) and serum hepcidin levels (Figure 2L) were lower in db/db mice, whereas hepcidin expression was significantly increased in KKAy mice (Figure 2M). Decreased hepcidin expression in db/db mice was ascribed to impaired leptin receptor-mediated signaling in previous studies.22,23 Regardless, the divergence in hepcidin expression implicates that hepcidin is not likely the primary mechanism of consistent Fpn upregulation in all 3 models of diabetes.
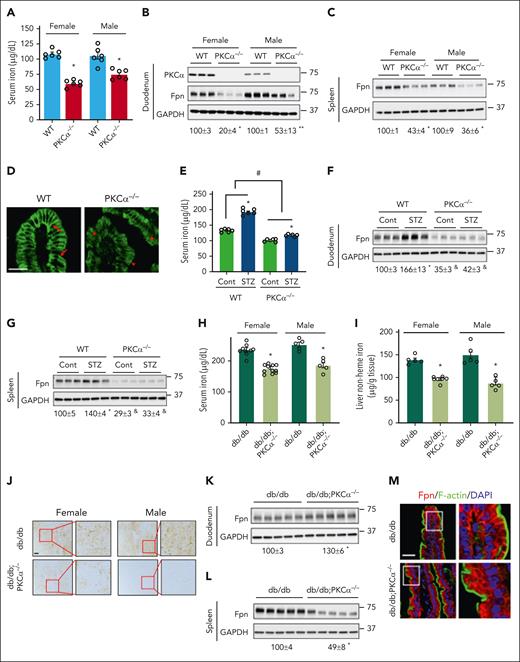
PKC is an important protein kinase that is activated by the hyperglycemia and mediates the pathogenesis of many diabetic complications.24 Impressively, the knockout of PKCα led to significantly decreased serum iron content (Figure 3A) and markedly decreased Fpn expression in the duodenum (Figure 3B) and spleen (Figure 3C) of both female and male mice. Confocal microscopy showed a decreased membrane but increased cytoplasmic expression of Fpn in PKCα−/− mice (Figure 3D). Consistently, splenic and duodenal iron contents, and duodenal FTH1 expression, were all increased in PKCα−/− mice (supplemental Figure 5). Importantly, PKCα knockout blunted the increases in serum iron (Figure 3E) and Fpn expression in STZ-induced diabetic mice in both the duodenum (Figure 3F) and spleen (Figure 3G). This finding is in line with our previous report of decreased liver iron content in STZ-treated PKCα−/− mice.17 These effects of PKCα deficiency were not dependent on changes in hepcidin expression or transcriptional regulation of Fpn in the duodenum or spleen (supplemental Figure 6).
Deficiency in PKCα downregulates Fpn expression in physiology and diabetes. (A) Serum iron and Fpn expression in the (B) duodenum and (C) spleen were determined in 16-week-old female and male PKCα−/− vs WT mice. Immunoblotting for PKCα shows the successful deletion of PKCα in PKCα−/− mice. (D) Representative confocal images show the localization of Fpn in the duodenum of WT and PKCα−/− male mice. Arrow, membrane Fpn expression; arrowhead, intracellular Fpn. (E) Serum iron and Fpn expression in the (F) duodenum and (G) spleen were determined in WT and PKCα−/− male mice with and without STZ-induced diabetes. (H) Serum iron was determined in female and male db/db and db/db;PKCα−/− mice. Liver iron content was determined in db/db and db/db;PKCα−/− mice by (I) biochemical assay and (J) Perls iron staining. Fpn protein expression was determined in the (K) duodenum and (L) spleen of db/db and db/db;PKCα−/− female mice. (M) Confocal images showing Fpn (red) localization in the enterocytes of db/db and db/db;PKCα−/− mice, with F-actin staining (green) as a marker of the cytoskeleton. Data are shown as mean ± SEM (n = 5-10). ∗∗P < .05 and ∗P < .01 compared with their WT controls. #P < .01 comparing the percentages of differences between WT and PKC−/− mice. &P < .01 compared with the WT control (WT-Cont) group. Bars, 50 μm.
Deficiency in PKCα downregulates Fpn expression in physiology and diabetes. (A) Serum iron and Fpn expression in the (B) duodenum and (C) spleen were determined in 16-week-old female and male PKCα−/− vs WT mice. Immunoblotting for PKCα shows the successful deletion of PKCα in PKCα−/− mice. (D) Representative confocal images show the localization of Fpn in the duodenum of WT and PKCα−/− male mice. Arrow, membrane Fpn expression; arrowhead, intracellular Fpn. (E) Serum iron and Fpn expression in the (F) duodenum and (G) spleen were determined in WT and PKCα−/− male mice with and without STZ-induced diabetes. (H) Serum iron was determined in female and male db/db and db/db;PKCα−/− mice. Liver iron content was determined in db/db and db/db;PKCα−/− mice by (I) biochemical assay and (J) Perls iron staining. Fpn protein expression was determined in the (K) duodenum and (L) spleen of db/db and db/db;PKCα−/− female mice. (M) Confocal images showing Fpn (red) localization in the enterocytes of db/db and db/db;PKCα−/− mice, with F-actin staining (green) as a marker of the cytoskeleton. Data are shown as mean ± SEM (n = 5-10). ∗∗P < .05 and ∗P < .01 compared with their WT controls. #P < .01 comparing the percentages of differences between WT and PKC−/− mice. &P < .01 compared with the WT control (WT-Cont) group. Bars, 50 μm.
PKCα knockout in db/db mice also led to significant reductions in serum (Figure 3H) and liver iron contents (Figure 3I-J) but not in blood glucose level (supplemental Figure 7). To our surprise, Fpn expression was increased in the duodenum in db/db;PKCα−/− mice (Figure 3K), despite a significant decrease in the spleen (Figure 3L). A closer examination by confocal microscopy showed that Fpn was largely found in the cytoplasm in the enterocytes of db/db;PKCα−/− mice (Figure 3M). PKCα deficiency in db/db mice did not alter hepcidin expression, nor Fpn mRNA expression in the duodenum or spleen (supplemental Figure 8A-C). Because of cytoplasmic expression of Fpn, iron was sequestered in the enterocytes of db/db;PKCα−/− mice (supplemental Figure 8D). Surprisingly, despite markedly decreased splenic expression of Fpn, iron content and FTH1 expression were lower in the spleen of db/db;PKCα−/− mice (supplemental Figure 8E-F). Our findings suggest the regulation of iron metabolism is more complex in db/db mice and that it requires further attention.
Collectively, these results highlight that PKCα is an important positive regulator of Fpn in the physiological setting and diabetic conditions.
PKCα decreases endocytic yet increases exocytotic trafficking of Fpn
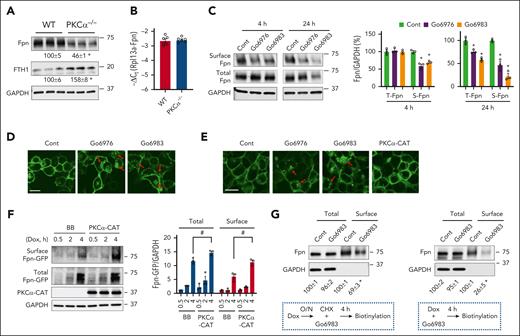
Next, we determined how Fpn is regulated by PKCα. By using cultured bone marrow–derived macrophages (BMDMs), we showed a significant decrease in Fpn protein and an increase in FTH1 in PKCα−/− BMDMs compared with the WT controls (Figure 4A). Again, Fpn mRNA expression was not altered by lacking PKCα (Figure 4B). The role of PKC was further explored by using Go6976, an inhibitor of PKCα, and Go6983, a pan-PKC inhibitor. In the acute phase (4 hours) of treatment, neither inhibitor altered total Fpn expression, but both decreased membrane Fpn expression (Figure 4C). A prolonged treatment (24 hours) reduced the total and membrane Fpn expression by both inhibitors, with a more prominent decrease in the membrane pool (Figure 4C).
PKCα decreases endocytic membrane targeting and increases exocytotic membrane targeting of Fpn. (A) The protein expression of Fpn and FTH1 was determined in BMDMs from WT and PKCα−/− mice. (B) Fpn mRNA expression was determined in WT and PKCα−/− BMDMs. (C) WT BMDMs were treated for 4 or 24 hours with 5 μM Go6976 or 2 μM Go6983, followed by biotin/streptavidin pulldown of cell surface proteins. The total and surface Fpn were then determined by blotting with anti-mouse Fpn antibody. (D) TRex cells were treated overnight with Dox (100 ng/mL) to induce Fpn-GFP expression, followed by a 2-hour pretreatment with cycloheximide (CHX, 100 μg/mL), an inhibitor of de novo protein synthesis, before treating with Go6976 or Go6983 for 4 hours. Subcellular localization of Fpn-GFP was then assessed by confocal microscopy. Red arrow denotes intracellular Fpn-GFP. (E) TRex cells were transduced with PKCα-CAT, or pretreated with Go6976 or Go6983, before a 4-hour treatment with Dox (1 μg/mL) and confocal imaging. (F) TRex cells transfected with backbone (BB) or PKCα-CAT were treated with Dox (1 μg/mL) for 0.5, 2 or 4 hours. Surface biotinylation was performed, and the total and surface Fpn-GFP expression was determined by blotting with anti-mouse GFP antibody. (G) TRex cells were treated as indicated underneath the blots. Both sets of cells were subjected to surface biotinylation at the end of 4-hour treatment by Go6983 or the control. Data shown are mean ± SEM of 3 independent experiments. ∗P < .01 compared with WT or the control conditions. #P < .05. Bar, 10 μm.
PKCα decreases endocytic membrane targeting and increases exocytotic membrane targeting of Fpn. (A) The protein expression of Fpn and FTH1 was determined in BMDMs from WT and PKCα−/− mice. (B) Fpn mRNA expression was determined in WT and PKCα−/− BMDMs. (C) WT BMDMs were treated for 4 or 24 hours with 5 μM Go6976 or 2 μM Go6983, followed by biotin/streptavidin pulldown of cell surface proteins. The total and surface Fpn were then determined by blotting with anti-mouse Fpn antibody. (D) TRex cells were treated overnight with Dox (100 ng/mL) to induce Fpn-GFP expression, followed by a 2-hour pretreatment with cycloheximide (CHX, 100 μg/mL), an inhibitor of de novo protein synthesis, before treating with Go6976 or Go6983 for 4 hours. Subcellular localization of Fpn-GFP was then assessed by confocal microscopy. Red arrow denotes intracellular Fpn-GFP. (E) TRex cells were transduced with PKCα-CAT, or pretreated with Go6976 or Go6983, before a 4-hour treatment with Dox (1 μg/mL) and confocal imaging. (F) TRex cells transfected with backbone (BB) or PKCα-CAT were treated with Dox (1 μg/mL) for 0.5, 2 or 4 hours. Surface biotinylation was performed, and the total and surface Fpn-GFP expression was determined by blotting with anti-mouse GFP antibody. (G) TRex cells were treated as indicated underneath the blots. Both sets of cells were subjected to surface biotinylation at the end of 4-hour treatment by Go6983 or the control. Data shown are mean ± SEM of 3 independent experiments. ∗P < .01 compared with WT or the control conditions. #P < .05. Bar, 10 μm.
A decrease in surface Fpn expression from PKC inhibition may result from accelerated internalization of the membrane Fpn and/or reduced exocytotic membrane targeting of the cytoplasmic Fpn. To address this question, we used TRex-Fpn–green fluorescent protein (GFP; TRex) cells that express Fpn-GFP upon doxycycline (Dox) induction. To determine whether PKC regulates Fpn endocytosis, TRex cells were treated Dox, followed by cycloheximide to block de novo protein synthesis, before treatments with Go6976 or Go6983. Both inhibitors induced a robust internalization of Fpn as indicated by the increased numbers of GFP-positive puncta in the cytoplasm (Figure 4D). This process was not dependent on Fpn ubiquitination (data not shown), a known mechanism for hepcidin-induced internalization of Fpn.25 Next, we determined whether PKC could also modulate the sorting of de novo synthesized Fpn to the membrane. TRex cells were pretreated with PKC inhibitors or transduced with PKCα-CAT, a constitutive active mutant of PKCα,26 before Dox treatment. Interestingly, cells pretreated by Go6976 or Go6983 displayed increased Fpn-GFP expression in the cytoplasm, with a larger effect by Go6983 (Figure 4E). Conversely, PKCα-CAT expression led to a significantly higher membrane Fpn-GFP expression by fluorescent imaging (Figure 4E) and surface biotinylation assay (Figure 4F). Moreover, while a 4-hour treatment by Go6983 induced an internalization of 31% membrane Fpn-GFP compared with the untreated control (Figure 4G, left), the same 4-hour treatment caused a 74% decrease in cell surface expression of de novo synthesized Fpn-GFP (Figure 4G, right). Altogether, these results demonstrate that PKCα (possibly also other PKC isoforms) regulates both the endocytic and exocytotic trafficking of Fpn.
PKCα counteracts hepcidin-induced ubiquitination and internalization of Fpn
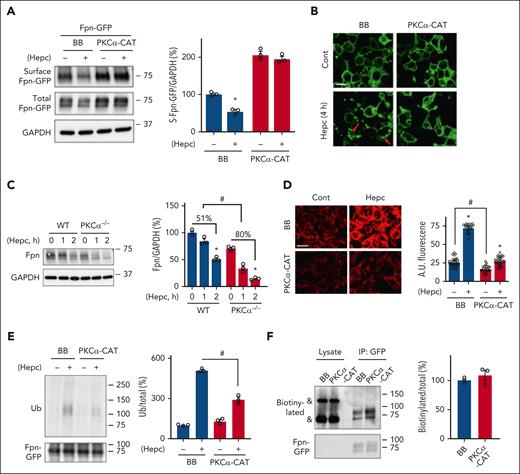
We also asked whether PKCα regulates Fpn sensitivity to hepcidin. Strikingly, PKCα-CAT expression almost completely blocked hepcidin-induced endocytosis of Fpn (Figure 5A). PKCα-CAT also increased baseline membrane expression of Fpn-GFP, consistent with our earlier finding in TRex cells. Two GFP antibody–recognized bands were noted in the total lysates (Figure 5A). They both represent Fpn-GFP as neither was present in the control transfection (supplemental Figure 9A). By comparing the pattern of Fpn-GFP in the total and surface pools, we could appreciate that only the larger but not the smaller size appeared in the membrane pool (supplemental Figure 9B). The smaller sized protein could be immature or newly synthesized Fpn. The blocking effect of PKCα on hepcidin-induced Fpn internalization was confirmed by confocal imaging (Figure 5B). Conversely, deficiency in PKCα potentiated hepcidin-induced degradation of Fpn in BMDMs (Figure 5C). Inhibition of PKCα with Go6976 also enhanced hepcidin-induced Fpn internalization in 293T cells (supplemental Figure 10). Moreover, PKCα-CAT expression reduced intracellular iron content in the basal condition, and mitigated hepcidin induced iron accumulation (Figure 5D), as determined by staining with FerroOrange,27,28 a fluorescent probe that recognizes labile Fe2+. This effect is likely attributable to the stimulatory role of PKCα in membrane Fpn expression and iron efflux. Of note, expression of PKCδ-CAT also abrogated hepcidin induced Fpn-GFP internalization (supplemental Figure 11).
PKCα counteracts hepcidin-induced ubiquitination and internalization of Fpn. 293T cells were transfected with Fpn-GFP together with BB or PKCα-CAT. The transfected cells were then treated for 4 hours with and without 0.5 μg/mL human hepcidin (Hepc). (A) Surface Fpn expression and (B) subcellular Fpn localization were determined by surface biotinylation assay and confocal microscopy, respectively. Surface Fpn-GFP was detected with 20% of the biotin/streptavidin pulldown from 300 μg total proteins. Red arrow denotes intracellular Fpn-GFP. (C) WT and PKCα−/− BMDMs were treated for 1 or 2 hours with 0.25 μg/mL mouse hepcidin, and Fpn expression was determined by immunoblotting with anti-mouse Fpn antibody. (D) 293T cells were transfected with BB or PKCα-CAT. The transfected cells were treated for 24 hours with and without 0.5 μg/mL human hepcidin, followed by determining intracellular labile Fe2+ content with 1 μM FerroOrange. The arbitrary fluorescent intensity was then quantified (n = 30 cells of 3 independent experiments). (E) TRex cells were transfected with BB or PKCα-CAT, and the expression of Fpn-GFP was induced overnight by 100 ng/mL Dox. Dox-induced cells were/were not treated for 2 hours with 0.5 μg/mL hepcidin, followed by immunoprecipitation of Fpn-GFP with anti-rabbit GFP antibody. The ubiquitination level of Fpn-GFP was determined with anti-ubiquitin FK2 antibody, and the total amount of immunoprecipitated Fpn-GFP was determined with anti-mouse GFP antibody. (F) Dox-treated TRex cells that were transfected with BB or PKCα-CAT were incubated for 30 minutes with 3 μg/mL biotinylated human hepcidin. After immunoprecipitation with anti-rabbit GFP antibody, the abundance of coimmunoprecipitated biotinylated hepcidin was determined with horseradish peroxidase–conjugated streptavidin. “&” denotes nonspecific bands. Data are shown as mean ± SEM of 3 independent experiments. ∗P < .01 compared with the untreated controls; #P < .01. Bar, 10 μm.
PKCα counteracts hepcidin-induced ubiquitination and internalization of Fpn. 293T cells were transfected with Fpn-GFP together with BB or PKCα-CAT. The transfected cells were then treated for 4 hours with and without 0.5 μg/mL human hepcidin (Hepc). (A) Surface Fpn expression and (B) subcellular Fpn localization were determined by surface biotinylation assay and confocal microscopy, respectively. Surface Fpn-GFP was detected with 20% of the biotin/streptavidin pulldown from 300 μg total proteins. Red arrow denotes intracellular Fpn-GFP. (C) WT and PKCα−/− BMDMs were treated for 1 or 2 hours with 0.25 μg/mL mouse hepcidin, and Fpn expression was determined by immunoblotting with anti-mouse Fpn antibody. (D) 293T cells were transfected with BB or PKCα-CAT. The transfected cells were treated for 24 hours with and without 0.5 μg/mL human hepcidin, followed by determining intracellular labile Fe2+ content with 1 μM FerroOrange. The arbitrary fluorescent intensity was then quantified (n = 30 cells of 3 independent experiments). (E) TRex cells were transfected with BB or PKCα-CAT, and the expression of Fpn-GFP was induced overnight by 100 ng/mL Dox. Dox-induced cells were/were not treated for 2 hours with 0.5 μg/mL hepcidin, followed by immunoprecipitation of Fpn-GFP with anti-rabbit GFP antibody. The ubiquitination level of Fpn-GFP was determined with anti-ubiquitin FK2 antibody, and the total amount of immunoprecipitated Fpn-GFP was determined with anti-mouse GFP antibody. (F) Dox-treated TRex cells that were transfected with BB or PKCα-CAT were incubated for 30 minutes with 3 μg/mL biotinylated human hepcidin. After immunoprecipitation with anti-rabbit GFP antibody, the abundance of coimmunoprecipitated biotinylated hepcidin was determined with horseradish peroxidase–conjugated streptavidin. “&” denotes nonspecific bands. Data are shown as mean ± SEM of 3 independent experiments. ∗P < .01 compared with the untreated controls; #P < .01. Bar, 10 μm.
Ubiquitination is necessary for hepcidin-induced endocytosis of Fpn.25 Indeed, PKCα-CAT significantly mitigated hepcidin-induced but not the baseline ubiquitination of Fpn (Figure 5E). We suspected that hyperactive PKCα may attenuate hepcidin-induced Fpn ubiquitination through interfering with hepcidin binding to membrane Fpn. However, binding assay failed to show a significant difference in the amount of hepcidin bound by Fpn in the presence or absence of PKCα-CAT expression (Figure 5F). These data demonstrate that PKCα counteracts the negative effect of hepcidin on Fpn by attenuating Fpn ubiquitination but not through changing hepcidin-Fpn interaction.
Iron excess activates PKCα in the enterocytes and macrophages
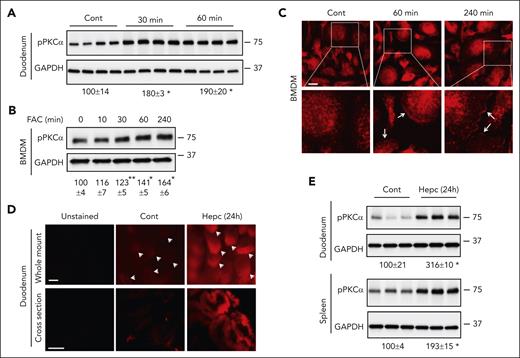
It is well known that intracellular iron accumulation induces changes in the expression of genes related to iron import, export, and storage dependent on the iron response element (IRE)-iron regulatory protein (IRP) system.29 We wondered whether iron excess induces the phosphorylation/activation of PKCα as a novel mechanism to facilitate iron efflux. We observed that oral administration of FeSO4 significantly increased the phosphorylation level of PKCα in the duodenum as early as 30 minutes (Figure 6A). A time course increase in the phosphorylation level of PKCα was also seen in cultured BMDMs treated by ferric ammonium citrate (Figure 6B). Confocal imaging showed a fraction of PKCα was associated with the plasma membrane in iron-loaded BMDMs (Figure 6C), suggesting the activation of PKCα. Hepcidin induces intracellular iron sequestration through the induction of Fpn degradation. Indeed, FerroOrange staining showed a robust increase in labile Fe2+ content in duodenal epithelium of hepcidin-treated mice (Figure 6D). A profound increase in PKCα phosphorylation was also noticed in the duodenum and the spleen (Figure 6E). Yet, we could not see altered phosphorylated PKCα expression in the liver of Hfe−/− mice (data not shown), a model of mild to moderate chronic iron overload. Our data thus demonstrate that PKCα is activated by excessive iron that, in turn, enhances Fpn-mediated iron efflux to mitigate intracellular iron overload.
Iron excess increases the phosphorylation and membrane localization of PKCα. (A) WT mice, after 4-hour starvation, received 50 mg/kg FeSO4 via gavage. After the indicated times, mice were euthanized, and villi were isolated for western blotting analysis of the phosphorylation of PKCα (pPKCα) with anti–phosphorylated-PKCα (Thr638) antibody. (B) BMDMs were treated with ferric ammonium citrate (FAC, 200 μM) for the indicated times, and the expression of pPKCα was determined. (C) BMDMs were treated with FAC, and the localization of PKCα was detected by staining with anti-PKCα antibody and then confocal imaging. White arrow, plasma membrane expression of PKCα. WT mice were treated with and without mouse hepcidin (20 μg per mouse) for 24 hours. (D) Labile Fe2+ content was determined in whole-mount duodenal tissue and the cross sections by staining with FerroOrange. White arrowhead, intestinal villous tip. The expression of pPKCα was analyzed in the duodenum and spleen by western blotting (E). Data are expressed as mean ± SEM of 3 independent experiments for panel B. ∗P < .01; and ∗∗P < .05 compared with the untreated controls. Bar, 10 μm for panel C or 100 μm for panel D.
Iron excess increases the phosphorylation and membrane localization of PKCα. (A) WT mice, after 4-hour starvation, received 50 mg/kg FeSO4 via gavage. After the indicated times, mice were euthanized, and villi were isolated for western blotting analysis of the phosphorylation of PKCα (pPKCα) with anti–phosphorylated-PKCα (Thr638) antibody. (B) BMDMs were treated with ferric ammonium citrate (FAC, 200 μM) for the indicated times, and the expression of pPKCα was determined. (C) BMDMs were treated with FAC, and the localization of PKCα was detected by staining with anti-PKCα antibody and then confocal imaging. White arrow, plasma membrane expression of PKCα. WT mice were treated with and without mouse hepcidin (20 μg per mouse) for 24 hours. (D) Labile Fe2+ content was determined in whole-mount duodenal tissue and the cross sections by staining with FerroOrange. White arrowhead, intestinal villous tip. The expression of pPKCα was analyzed in the duodenum and spleen by western blotting (E). Data are expressed as mean ± SEM of 3 independent experiments for panel B. ∗P < .01; and ∗∗P < .05 compared with the untreated controls. Bar, 10 μm for panel C or 100 μm for panel D.
The loss-of-function of PKCα attenuates iron overload in hereditary hemochromatosis
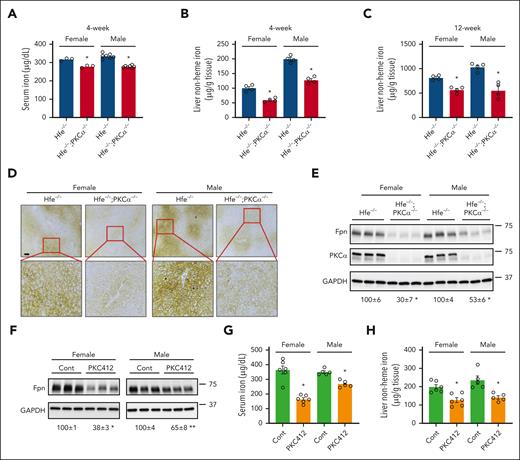
In the aforementioned, we showed that PKCα accounts for Fpn upregulation and iron overload in diabetes. We further evaluated whether PKC may be targeted to alleviate iron overload associated with hereditary hemochromatosis by using a commonly used hemochromatosis model, Hfe−/− mice. Compared with Hfe−/− mice, Hfe−/−;PKCα−/− mice displayed a significantly lower serum iron content in both female and male (Figure 7A). Likewise, liver iron content was significant lower in 4- (Figure 7B) and 12-week-old (Figure 7C) Hfe−/−;PKCα−/− mice. Decreased liver iron content in Hfe−/−;PKCα−/− mice was confirmed by Perls iron staining (Figure 7D). A lowered Fpn protein expression was also observed in the duodenum of Hfe−/−;PKCα−/− mice (Figure 7E). This decrease is not owing to hepcidin, because a decreased hepcidin expression was seen in Hfe−/−;PKCα−/− mice (supplemental Figure 12A). Fpn mRNA expression was not altered in Hfe−/−;PKCα−/− mice (supplemental Figure 12B-C), further supporting the posttranslational regulation of Fpn by PKCα. Consistent with the downregulated Fpn, iron accumulation was seen in the duodenum and spleen of Hfe−/−;PKCα−/− mice (supplemental Figure 12D-F).
The loss-of-function of PKCα mitigates iron overload in hereditary hemochromatosis. (A) Serum iron level was measured in female and male Hfe−/− and Hfe−/−; PKCα−/− mice (4 weeks old). Liver nonheme iron content was measured in female and male Hfe−/− and Hfe−/−; PKCα−/− mice at (B) 4 and (C) 12 weeks of age. (D) Perls iron staining shows iron deposition in the liver of female and male Hfe−/− and Hfe−/−; PKCα−/− mice. (E) Duodenal Fpn expression in female and male Hfe−/− and Hfe−/−; PKCα−/− mice by immunoblotting with anti-mouse Fpn antibody. (F-H) Female and male Hfe−/− mice were fed an iron-deficient diet from when they were born until 3 weeks of age. Mice were then transferred onto chow diet and receive PKC412 (50 mg/kg body weight) or vehicle for 2 weeks. (F) Duodenal Fpn expression and (G) serum and (H) liver iron content were determined. Data are expressed as mean ± SEM (n = 3-7). ∗P < .01; and ∗∗P < .05 compared with Hfe−/− mice or the corresponding controls. Bar, 100 μm.
The loss-of-function of PKCα mitigates iron overload in hereditary hemochromatosis. (A) Serum iron level was measured in female and male Hfe−/− and Hfe−/−; PKCα−/− mice (4 weeks old). Liver nonheme iron content was measured in female and male Hfe−/− and Hfe−/−; PKCα−/− mice at (B) 4 and (C) 12 weeks of age. (D) Perls iron staining shows iron deposition in the liver of female and male Hfe−/− and Hfe−/−; PKCα−/− mice. (E) Duodenal Fpn expression in female and male Hfe−/− and Hfe−/−; PKCα−/− mice by immunoblotting with anti-mouse Fpn antibody. (F-H) Female and male Hfe−/− mice were fed an iron-deficient diet from when they were born until 3 weeks of age. Mice were then transferred onto chow diet and receive PKC412 (50 mg/kg body weight) or vehicle for 2 weeks. (F) Duodenal Fpn expression and (G) serum and (H) liver iron content were determined. Data are expressed as mean ± SEM (n = 3-7). ∗P < .01; and ∗∗P < .05 compared with Hfe−/− mice or the corresponding controls. Bar, 100 μm.
PKC412 is a pharmacological drug that was initially identified as an inhibitor of PKC, and has been approved for treating acute myeloid leukemia, attributable to its inhibitory effects on kinases including FMS-like tyrosine kinase 3 receptor.30,31 By using PKC412, we determined whether inhibition of PKC could attenuate body iron loading in Hfe−/− mice. Intriguingly, PKC412 treatment for 2 weeks led to a significant decrease in duodenal Fpn expression in female and male Hfe−/− mice (Figure 7F). The serum (Figure 7G) and liver (Figure 7H) iron contents were also significantly decreased in PKC412-treated mice. These results demonstrate that targeting PKC could successfully mitigate Fpn expression and iron overload associated with hereditary hemochromatosis.
Discussion
In this work, we have demonstrated that the upregulation of Fpn accounts for the increased iron absorption and systemic iron overload in diabetes. Our findings showed that hepcidin is dispensable, and the hyperactive PKCα is responsible for diabetes induced Fpn upregulation. PKCα upregulates Fpn in both the enterocytes and macrophages, the 2 cell types that are critical for systemic iron homeostasis. Hyperactive PKCα not only enhances exocytotic mobilization of nascent Fpn to the plasma membrane but also attenuates the internalization of membrane expressed Fpn. PKCα also stabilizes the membrane expression of Fpn by attenuating hepcidin-induced Fpn ubiquitination.
Our findings of increased circulatory and hepatic iron contents in STZ-induced diabetes is consistent with previous reports.16,17 Elevated serum iron in our db/db mice is in line with previous observations by other groups.32,33 Increased hepatic iron loading is supported by a previous report of increased liver iron staining in db/db mice.34 In contrast, Altamura et al showed hepatic iron deficiency in db/db mice, despite the claimed increase in systemic iron content.35 The same finding was seen in our KKAy mice, which showed an elevation in serum iron but a deficiency in liver iron content. The reason for the discrepant findings in the db/db model is unclear but may be because of differences in hepatic expression or function of iron importers. Regardless, our study showed consistently increased Fpn expression across the 3 diabetic mouse models and in humans with diabetes. Patients who are diabetic can develop iron overload displaying increased serum and hepatic iron content,32,36 whereas a high prevalence (∼20%-30%) of anemia is also noted in the diabetic population.37 The mechanisms underlying diabetes-associated anemia remain unclear, but deficiency in erythropoietin production due to renal dysfunction in prolonged diabetes could be 1 cause.38,39 Consistent with the increased serum iron content and transferrin saturation in a previous report,32 our study identified that Fpn expression and iron efflux in enterocytes were higher in patients who are diabetic. Future work is needed to dissect the complex relationship between iron metabolism, anemia, and possibly their dependency on the progression of diabetes. Based on previous evidence and our current findings, we envisage that increased iron absorption with compromised iron use in red blood cell production would lead to increased tissue iron overload, aggravating tissue injury and diabetic complications.
The upregulation of Fpn was previously reported in diabetic rats,16 attributable to hepcidin deficiency dependent on the decreased expression of STAT3.40,41 Our results in 3 different diabetic models failed to suggest an important role of hepcidin in Fpn upregulation, particularly that hepcidin was increased in KKAy mice. Instead, our work identified a novel role of PKCα in sustaining Fpn expression in the enterocytes and macrophages, and in the control of systemic iron homeostasis. Many PKC members are activated in the hyperglycemia context and mediate the development of diabetic complications.24 In the intestine, PKCα is the predominant isoform and is activated in diabetes according to our previous studies.17,42 We previously showed that PKCα promotes luminal membrane expression of divalent metal transporter 1 and iron uptake in the enterocytes of diabetic mice.17 Other studies have suggested a role of PKC in upregulating glucose transporters including SGLT1 and GLUT2 in the intestine through stabilized expression at the plasma membrane.43
The mechanisms of Fpn regulation by PKCα are multifold but independent of hepcidin. In the resting condition, PKCα maintains a counterbalance between the endocytic and exocytotic trafficking of Fpn. Compromised expression or activity of PKCα enhances the internalization and reduces membrane targeting of Fpn. Conversely, hyperactivation of PKCα represses Fpn internalization and promotes exocytotic trafficking of the cytoplasmic Fpn. This role of PKCα in the basal state is not through changes in Fpn ubiquitination, a necessary step in hepcidin-induced internalization and degradation of Fpn.25 Upon activation, PKC undergoes cytosol-to-membrane translocation,44 and regulates remodeling of the cytoskeleton filaments.45 Thus, it is possible that activated PKCα modulates cytoskeleton dynamics and/or promotes Fpn interaction with the cytoskeletal network. This speculation is supported by our recent proteomic analysis that Fpn is potentially bound by multiple PDZ domain proteins (P. He, unpublished data, July 2023), which are important scaffold proteins that connect membrane proteins with the cytoskeleton.46 It was also reported that PKC increases membrane expression of occludin47 and transporter GLUT148 in a phosphorylation-dependent manner. The possibilities that PKCα regulates cytoskeletal remodeling and phosphorylation-dependent trafficking and membrane stabilization of Fpn remain to be interrogated in future work.
In hepcidin-exposed conditions, ubiquitination of Fpn is crucial for its internalization and subsequent downregulation.25 Our study identified that activation of PKCα strongly protected Fpn from hepcidin-induced ubiquitination and internalization. This effect was not through changing the interaction between hepcidin and Fpn. We have not determined the detailed mechanism in the present study but 1 potential mechanism is that activation of PKCα disrupts the assembly of ubiquitination machinery complex involving E1 enzyme UBA649 and E3 ligase RNF217,50 which were reported to mediate Fpn ubiquitination.
Aside from our finding of a novel role of PKCα in Fpn upregulation and iron export, we have an intriguing observation that PKCα is activated when intracellular iron is in excess. This probably represents a novel protective mechanism that cells evolved to dispose of excessive iron, particularly labile iron, which causes oxidative damage to the cellular contents. Iron-mediated activation of PKCα may share a similar mechanism with that in diabetes, both are associated with the increased production of reactive oxygen species that potentially activates PKC.51,52 The PKC-mediated upregulation of Fpn is potentially a new mechanism in addition to the well-characterized IRE-IRP system29 and hypoxia-inducible factor 253 in the regulation of Fpn.
In conclusion, we demonstrate, to our knowledge, for the first time, that PKCα is a novel regulator of Fpn, and PKCα-dependent upregulation of Fpn is the primary mechanism underlying diabetes-induced iron overload. We have also shown that the knockout or pharmacological inhibition of PKCα attenuates Fpn-dependent systemic iron overload. Our findings of the importance of PKCα for Fpn regulation opened a new avenue for the control of systemic and cellular iron homeostasis in physiological and pathological conditions. The limitation of our study is that PKCα regulates a spectrum of proteins and, therefore, inhibition of PKCα potentially alters other physiological processes. Future work toward a better understanding of the PKCα–Fpn regulatory axis may lead to the identification of novel and specific target(s) in the control of iron homeostasis.
Acknowledgments
This study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK125647 (to P.H.). This work was supported by the Emory University Integrated Cellular Imaging Core Facility (Research Resource Identifier: SCR_023534).
Authorship
Contribution: S.B., H.T., and P.H. conducted the experiments; S.L. screened and provided human duodenal samples; S.B. and I.W. performed data analysis; S.B., E.N., and P.H. designed the experiments and wrote the manuscript; and S.L., A.J., S.S., E.N., and P.H. edited the manuscript.
Conflict-of-interest disclosure: E.N. is a shareholder and scientific adviser of Intrinsic LifeSciences and Silarus Therapeutics; and is a consultant for Disc Medicine, Ionis Pharmaceuticals, Protagonist, GlaxoSmithKline, and Vifor. The remaining authors declare no competing financial interests.
Correspondence: Peijian He, Division of Digestive Diseases, Department of Medicine, Emory University School of Medicine, 615 Michael St, Atlanta, GA 30322; email: phe3@emory.edu.
References
Author notes
All data have been provided in this manuscript. For raw data, please contact the correspondence author, Peijian He (phe3@emory.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal