The mortality from herpesvirus–associated malignancies, such as Kaposi sarcoma (KS), may primarily be due to cellular inflammasome activation and systemic cytokine upregulation from the underlying KS-associated disorders (KAD) according to Lage et al1 in this issue of Blood. KAD is a collection of KS-concurrent severe diseases that include primary effusion lymphoma, KS, a plasmablastic form of multicentric Castleman disease, KS inflammatory cytokine syndrome, and generalized responses to abnormally high virus replication.2
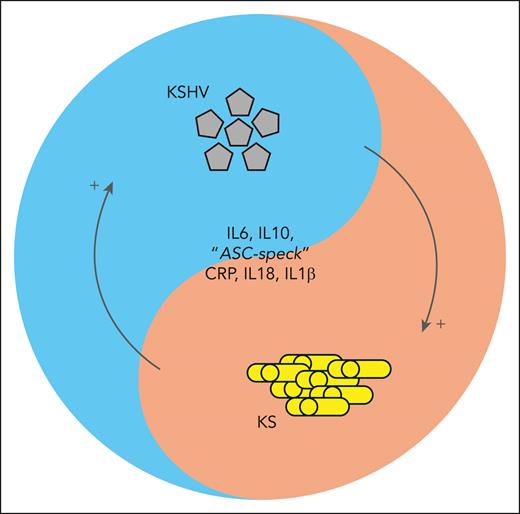
Patients with KS and KAD, as well as some patients with KS without a histopathological confirmation of KAD, present with abnormally high cytokine levels and viremia. A selective cytokine storm, chiefly due to interleukin-6 (IL-6) and IL-10, has been reported for HIV KS in children and adults.3,4 Lage et al add IL-18 and IL-1β to the list of potential serum biomarkers for impending patient deterioration due to concurrent KAD (see figure). They also found that monocyte-associated caspase-1/4/5 activity is increased and associated with the elevation of IL-18 and IL-1β during intracellular inflammasome activation.
Schema highlighting the interaction between inflammation, the virus, and the cancer. ASC, apoptosis-associated speck-like protein containing a CARD; CRP, C-reactive protein.
Schema highlighting the interaction between inflammation, the virus, and the cancer. ASC, apoptosis-associated speck-like protein containing a CARD; CRP, C-reactive protein.
Kaposi sarcoma herpesvirus (KSHV) viral load levels correlate with cytokine responses in some studies but not in others. There is no Food and Drug Administration–approved test for KSHV. Some patients with KS present with high viral loads, and others have no detectable virus. Yet both groups have similar clinical presentations.5 The simple one-way frameworks of virus-to-inflammation-to-disease or virus-to-disease-to-inflammation and cytokine release do not seem to apply here or to tumor virus infections in general. COVID-19, Ebola virus disease, and other diseases driven by acute virus infections and their corresponding inflammatory responses are the wrong paradigm for understanding delayed onset viral cancers, although “long COVID” may offer some pointers.
Parsing patients with KS into low- and high-risk groups, that is, into those that do well on HIV-suppressive therapy alone, those that need immediate intensive chemotherapy, and those that would benefit from immune modulatory therapy alone, represents the next frontier in KS management for people living with HIV (PLWH). The recognition that “cancer is not one disease, but many” marked the transition from purely cytotoxic therapy to tailored regimens and targeted drugs. Similarly, “KS is not one disease, but many” will transform the next 20 years of KS treatment if we can predict who falls into which category. The field needs a “sorting hat” for KS, a nomogram to assist experience and intuition.
This current report highlights the importance of studying outliers systematically. The machine learning community calls them “edge cases.” KAD could be understood as a collection of edge cases. Others would be PLWH, who present with KS despite undetectable HIV viral load, or those with coincident, virus-driven lymphoproliferation.6 Those edge cases are not rare in KS. Pediatric KS often presents devoid of any skin lesions. No one knows if there are unrecognized, underlying genetic determinants as well that predispose certain patients but not others to KAD. Identifying edge cases by a routine blood test(s) for intensified observation and follow-up may be the most immediate impact of the study by Lage et al on clinical practice, particularly in low- and middle-income countries where advanced diagnostics and intensive care options are limited. Here, choice is compelled by necessity, not rationality,
Today, KS is perceived very differently than in 1994 when KSHV was discovered and the AIDS epidemic lacked HIV treatments. Today, KS remains the most common cancer in PLWH, with 5-year survival climbing to ∼80% if patients have access to liposomal doxorubicin. Many do not have access to this drug, however, as they are part of underserved populations or reside in low- and middle-income countries.5,7 A fraction of patients with KS fail cytotoxic and antiretroviral therapy entirely, another fraction are cured by immune checkpoint inhibitor therapy, and another fraction are cured by broadly cytokine-stymieing agents such as oral pomalidomide.8 Oral etoposide may be considered an anti-inflammation agent, as adding it reduces immune reconstitution inflammatory syndrome in KS patients.9 If modulating viral neoplasia modulates virus-induced inflammation, how well would the reverse work?
The current lack of mechanistic understanding in KS is a limitation that plagues the rational combination of antitumor and anti-inflammation drugs. Forty years after the discovery of KSHV, there is no single patient-derived cell line to test the effect of immune modulatory agents on the virus or the cancer. If there was one, how well would a cell line replicate the cytokine cross talk within the tumor environment? The field is on thin ice, trying to link patient responses to established theories about inflammation. We have more technology at our disposal than ever before, but without a molecularly defined basis for decision-making, how far can biomarker correlations alone inform clinical practice? “KS is not one disease, but many” will be a curse and an opportunity for the next 40 years of KS research.
Conflict-of-interest disclosure: D.P.D. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal