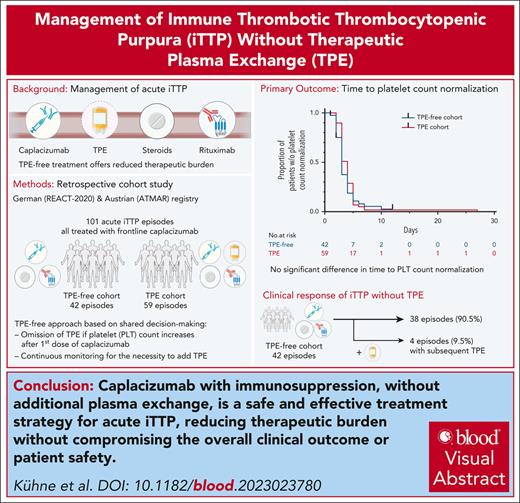
Key Point
Caplacizumab with immunosuppression, but without additional TPE, is an effective and safe treatment strategy for acute iTTP.
Visual Abstract
Immune thrombotic thrombocytopenic purpura (iTTP) is a rare, life-threatening autoimmune disorder caused by a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13) deficiency. Caplacizumab, an anti–von Willebrand factor nanobody, is approved for iTTP treatment, reducing the need for therapeutic plasma exchange (TPE) and improving platelet count recovery and survival. We conducted a retrospective study on 42 acute iTTP cases in Austria and Germany, treated with a modified regimen aimed at avoiding TPE if platelet count increased after the first caplacizumab dose. Baseline characteristics and patient outcomes were compared with a control group of 59 patients with iTTP receiving frontline treatment with TPE, caplacizumab, and immunosuppression. The main outcome was the time to platelet count normalization. Secondary outcomes included clinical response, exacerbation, refractory iTTP, iTTP-related deaths, and the time to platelet count doubling. The median time to platelet count normalization was similar between the 2 cohorts (3 and 4 days; P = .31). There were no significant differences in clinical response, exacerbations, refractoriness, iTTP-related deaths, or time to platelet count doubling, reflecting the short-term treatment response. Four patients did not respond to the first caplacizumab dose, and TPE was subsequently initiated. Cytomegalovirus infection, HIV/hepatitis B virus coinfection, an ovarian teratoma with associated antiplatelet antibodies, and multiple platelet transfusions before the correct diagnosis may have impeded the immediate treatment response in these patients. In conclusion, caplacizumab and immunosuppression alone, without TPE, rapidly controlled thrombotic microangiopathy and achieved a sustained clinical response in iTTP. Our study provides a basis for TPE-free iTTP management in experienced centers via shared decision-making between patients and treating physicians.
Introduction
Immune thrombotic thrombocytopenic purpura (iTTP) is an ultrarare, acute, and life-threatening autoimmune disease. Therapeutic plasma exchange (TPE), along with steroids and rituximab, has been used to address the underlying deficiency of a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS13) activity, which is central to iTTP pathophysiology. The approval of the anti–von Willebrand factor (VWF) nanobody caplacizumab has added a new principle to acute iTTP management. Caplacizumab prevents fatal microthrombus formation and subsequent organ ischemia, leading to faster platelet count normalization and a reduced incidence of adverse events, such as iTTP-related death, iTTP recurrence, and major thromboembolic events.1-3 Caplacizumab has been approved for the treatment of acute iTTP episodes in combination with TPE and immunosuppression. However, its optimal integration into clinical practice remains an ongoing topic of discussion. Current guidelines recommend the use of caplacizumab over withholding it and contend that early administration in the disease course confers the greatest benefits.4,5
Since caplacizumab’s approval, a growing number of reports have suggested the possibility of managing acute iTTP episodes without TPE, given the rapid termination of thrombotic microangiopathy features through anti-VWF therapy.6-9 Certain clinical scenarios, such as allergic reactions to plasma products, patient refusal of blood products, or a rapid and strong platelet count response to the initial caplacizumab dose, may allow withholding TPE.
TPE-free management of acute iTTP episodes offers several advantages, such as reduced technical and logistical complexity, avoidance of central venous catheter placement and adverse effects of donor plasma (eg, allergic reactions, hypotension, and infections), and improved patient convenience. However, the potential benefits of TPE, which has been the first choice for iTTP management since 1991, must also be carefully considered.10 Rapid removal of ADAMTS13 inhibitory autoantibodies, ultralarge VWF multimers, and ADAMTS13 immune complexes, as well as immediate replacement of ADAMTS13 and VWF with a normal multimeric composition, cannot be achieved with caplacizumab and immunosuppression alone. Additional effects of TPE, such as restoration of increased cytokine levels or potentially harmful boosting of autoantibodies, may also be considered, although they are less well characterized.11-13
This study evaluates the treatment of acute iTTP with caplacizumab and immunosuppression without TPE in comparison to management with TPE, caplacizumab, and immunosuppression. The objective of this modified treatment regimen was to reduce the therapeutic burden without compromising the overall clinical outcome or patient safety.
Methods
Patient population and study design
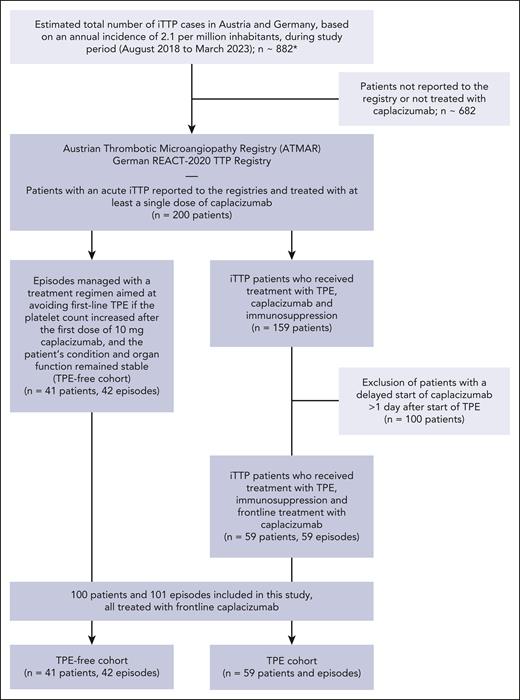
This was a retrospective cohort study. All patients in this study were identified from the Austrian Thrombotic Microangiopathy Registry and the German REACT-2020 TTP registry. The registry studies were approved by the local ethics committees (REACT-2020, registered at www.ClinicalTrials.gov as NCT04985318). Details of patient selection are shown in Figure 1 (study flow diagram). All cases with the intention to treat without TPE reported to our registries were included in this study. Parts of this cohort have been published previously.7,14-17
Study flow diagram detailing patient selection. ∗Total number of iTTP cases during study period was estimated given a population of 90 million inhabitants (for Austria and Germany), and an annual incidence of 2.1 per million, as identified in Miesbach et al.26
Study flow diagram detailing patient selection. ∗Total number of iTTP cases during study period was estimated given a population of 90 million inhabitants (for Austria and Germany), and an annual incidence of 2.1 per million, as identified in Miesbach et al.26
A total of 42 acute iTTP episodes in 41 patients were managed with a treatment regimen aimed at avoiding first-line TPE (TPE-free cohort). TPE-free management of patients was performed at 9 medical centers in Germany and 5 medical centers in Austria between 2019 and 2023. A comprehensive list of all treatment centers involved in TPE-free patient management can be found in the supplemental Appendix (supplemental Table 1), available on the Blood website. At these treatment centers patients with iTTP were consecutively approached for TPE-free management.
Demographic and baseline disease characteristics, treatment modalities, and patient outcomes of this cohort were compared with a control group of 59 patients with iTTP who received frontline treatment with caplacizumab, in addition to TPE and immunosuppression, which is the standard of care for iTTP in Austria and Germany (TPE cohort). Frontline caplacizumab treatment was the sole criterion for patient selection from the Austrian Thrombotic Microangiopathy Registry and REACT-2020 TTP registry to the control group. Frontline use of caplacizumab was defined as the initiation of caplacizumab together with TPE once an iTTP diagnosis was suspected. Patients in the control cohort were treated at 22 medical centers in Austria and Germany between 2018 and 2023. A comprehensive list of all treatment centers involved in patient management can be found in the supplemental Appendix.
All patients received at least daily checks of complete blood count (CBC) and serum chemistry to monitor the treatment response. ADAMTS13 activity testing was done weekly in most of the patients. Time points for ADAMTS13 activity measurements were at the discretion of the treating physicians. For measurement of ADAMTS13 activity and quantification of anti-ADAMTS13 antibodies, various methods were used as locally available (ie, chromogenic enzyme-linked immunosorbent assay techniques, fluorescence resonance energy transfer assay FRETS-VWF73, or anti-ADAMTS13 immunoglobulin G antibody enzyme-linked immunosorbent assay or Bethesda-assay, respectively). Laboratory parameters (blood cell counts, serum chemistry) were measured with local standard methods.
Intensive care unit (ICU) admissions were based on patients' need for life support, monitoring requirements, and the need for TPE, which is customarily administered on ICUs in Austria and Germany.
TPE-free management of acute iTTP
A TPE-free treatment regimen for acute iTTP episodes was implemented based on shared decision-making and consideration of patients’ preferences after a thorough discussion of iTTP treatment modalities, including TPE as the standard of care. Patients with neurological involvement upon admission were included if shared decision-making was not affected, as adjudicated by the local medical team. This was viable either because the symptoms were relatively mild or because the symptoms had improved after the initial intravenous administration of caplacizumab.
There was no uniform protocol for TPE-free patient management with regards to the timing of additional CBC checks and the second caplacizumab dose. Patients on TPE-free management received additional CBC checks within hours after the first dose of caplacizumab, at the discretion of the responsible physicians. CBC checks were performed at least within 24 hours after treatment initiation. The timing of the second caplacizumab dose was at the discretion of the medical team, with the latest application within 24 hours after treatment initiation. The need to add TPE was continuously monitored in all patients on a TPE-free regimen.
The criteria to withhold TPE and to proceed with TPE-free patient management after the first dose of 10 mg caplacizumab were an increasing platelet count, a stable or improving clinical condition, and stable or improving parameters of organ damage. Any increase in the platelet count beyond the local laboratory measurement variation was acceptable, regardless of the absolute count. The decision to add TPE was made by the responsible medical team. Parameters to guide subsequent initiation of TPE were a missing increase or even a decrease in platelet count, a worsening clinical condition, or progressing or new organ damage.
Outcome parameters
The primary outcome parameter was the time to platelet count normalization (platelet count ≥150 × 109/L). Key secondary outcomes were the proportions of patients achieving a clinical response, experiencing clinical exacerbation, developing refractory TTP, the number of TTP-related deaths, and the time to platelet count doubling. Other secondary outcomes included the time to recovery of ADAMTS13 activity to ≥20% after treatment initiation, duration of hospital stay, proportion of patients admitted to an ICU, and duration of ICU stay per episode. We refrained from comparing any organ damage markers between groups, which may have been affected by TPE.
Clinical response, exacerbation, remission, and relapse were evaluated according to current outcome definitions as revised by the International Working Group for TTP in 2021.18 Refractory TTP was evaluated according to the most recent definition: persistent thrombocytopenia, lack of a sustained platelet count increment or platelet count of <50 × 109/L, and a persistently elevated lactate dehydrogenase (LDH) level (>1.5 × upper limit of normal) despite 5 days of TPE.19 Retrospective safety assessments with an evaluation of complications were performed in both cohorts. Major bleeding was graded using the International Society on Thrombosis and Haemostasis grading system.20
Statistical analysis
R statistics version 4.2.3 was used for statistical analysis. GraphPad Prism version 10.0.0 was used for the compilation of graphs (GraphPad Software, San Diego, CA). For statistical comparison of continuous data, 2-tailed Student t tests or Mann-Whitney U tests were used, whereas categorical data were compared using the Fisher exact test. A 2-sided log-rank test was performed based on a Kaplan-Meier analysis for the time to platelet count normalization, the time to platelet count doubling, and the time to recovery of ADAMTS13 activity to ≥20%. Statistical significance was set at P < .05.
Results
Demographic and baseline disease characteristics
The demographic and baseline clinical features of the TPE-free and TPE cohorts at the initial presentation are shown in Table 1. The parameters of the initial clinical presentation did not differ between the 2 groups, except for a significantly higher initial LDH level in the TPE group (median, 703 vs 1052 U/L; P < .01). ADAMTS13 activity at baseline was <10% in all patients except for 1 in the TPE cohort. In this patient, the diagnosis of iTTP was based on a known history of iTTP, thrombocytopenia, and microangiopathic hemolytic anemia at baseline.
Demographic and baseline disease characteristics of the TPE-free and TPE cohorts treated with frontline caplacizumab
| Parameter . | TPE-free cohort (n = 42) . | TPE cohort (n = 59) . | P value . |
|---|---|---|---|
| Median age (range), y | 43 (20-83) | 47 (20-80) | .43 |
| Female/male sex | .82 | ||
| n (%) | 31 (75.6)/10 (24.4) | 40 (67.8)/17 (28.8) | |
| Data missing, n (%) | 0 (0) | 2 (3.4) | |
| Mean BMI (range), kg/m2 | 29 (17-50) | 28 (20-46) | .62 |
| First iTTP episode/iTTP relapse | .40 | ||
| n (%) | 25 (59.5)/17 (40.5) | 38 (64.4)/18 (30.5) | |
| Data missing, n (%) | 0 (0) | 3 (5.1) | |
| Median platelet count (range), × 109/L | 16 (4-127) | 12 (3-52) | .09 |
| Median LDH (range), U/L | 703 (214-2500) | 1052 (373-3467) | <.01 |
| Initial troponin | .17 | ||
| Elevated, n (%) | 16 (38.1) | 27 (45.8) | |
| Not elevated, n (%) | 11 (26.2) | 8 (13.6) | |
| Data missing, n (%) | 15 (35.7) | 24 (40.7) | |
| Median serum creatinine (range), mg/dL | 0.95 (0.57-5.30) | 1.07 (0.5-3.65) | .07 |
| ADAMTS13 activity <10%, n (%) | 42 (100) | 58 (98.3)∗ | >.99 |
| Glasgow coma scale, n (%) | .17 | ||
| 15 | 38 (90.5) | 43 (72.9) | |
| <15 | 4 (9.5) | 11 (18.6) | |
| Data missing | 0 (0) | 5 (8.5) | |
| Neurological symptoms upon admission, n (%) | |||
| Patients with at least 1 neurological symptom | 24 (57.1) | 26 (44.1) | .23 |
| Aphasia/dysarthria | 4 (9.5) | 8 (13.6) | |
| Blurred vision | 4 (9.5) | 3 (5.1) | |
| Coma | 0 (0) | 2 (3.4) | |
| Headache | 7 (16.7) | 4 (6.8) | |
| Focal deficiency | 4 (9.5) | 5 (8.5) | |
| Paresthesia | 4 (9.5) | 3 (5.1) | |
| Seizure | 1 (2.4) | 3 (5.1) | |
| Confusion | 8 (19.0) | 10 (16.9) | |
| French severity score, n (%) | .90 | ||
| Low (0-1) | 27 (64.3) | 30 (50.8) | |
| Intermediate (2) | 10 (23.8) | 15 (25.4) | |
| High (3-4) | 5 (11.9) | 6 (10.2) | |
| Data missing | 0 (0) | 8 (13.6) |
| Parameter . | TPE-free cohort (n = 42) . | TPE cohort (n = 59) . | P value . |
|---|---|---|---|
| Median age (range), y | 43 (20-83) | 47 (20-80) | .43 |
| Female/male sex | .82 | ||
| n (%) | 31 (75.6)/10 (24.4) | 40 (67.8)/17 (28.8) | |
| Data missing, n (%) | 0 (0) | 2 (3.4) | |
| Mean BMI (range), kg/m2 | 29 (17-50) | 28 (20-46) | .62 |
| First iTTP episode/iTTP relapse | .40 | ||
| n (%) | 25 (59.5)/17 (40.5) | 38 (64.4)/18 (30.5) | |
| Data missing, n (%) | 0 (0) | 3 (5.1) | |
| Median platelet count (range), × 109/L | 16 (4-127) | 12 (3-52) | .09 |
| Median LDH (range), U/L | 703 (214-2500) | 1052 (373-3467) | <.01 |
| Initial troponin | .17 | ||
| Elevated, n (%) | 16 (38.1) | 27 (45.8) | |
| Not elevated, n (%) | 11 (26.2) | 8 (13.6) | |
| Data missing, n (%) | 15 (35.7) | 24 (40.7) | |
| Median serum creatinine (range), mg/dL | 0.95 (0.57-5.30) | 1.07 (0.5-3.65) | .07 |
| ADAMTS13 activity <10%, n (%) | 42 (100) | 58 (98.3)∗ | >.99 |
| Glasgow coma scale, n (%) | .17 | ||
| 15 | 38 (90.5) | 43 (72.9) | |
| <15 | 4 (9.5) | 11 (18.6) | |
| Data missing | 0 (0) | 5 (8.5) | |
| Neurological symptoms upon admission, n (%) | |||
| Patients with at least 1 neurological symptom | 24 (57.1) | 26 (44.1) | .23 |
| Aphasia/dysarthria | 4 (9.5) | 8 (13.6) | |
| Blurred vision | 4 (9.5) | 3 (5.1) | |
| Coma | 0 (0) | 2 (3.4) | |
| Headache | 7 (16.7) | 4 (6.8) | |
| Focal deficiency | 4 (9.5) | 5 (8.5) | |
| Paresthesia | 4 (9.5) | 3 (5.1) | |
| Seizure | 1 (2.4) | 3 (5.1) | |
| Confusion | 8 (19.0) | 10 (16.9) | |
| French severity score, n (%) | .90 | ||
| Low (0-1) | 27 (64.3) | 30 (50.8) | |
| Intermediate (2) | 10 (23.8) | 15 (25.4) | |
| High (3-4) | 5 (11.9) | 6 (10.2) | |
| Data missing | 0 (0) | 8 (13.6) |
All parameters are presented per episode, except for sex. The TPE-free cohort comprised 42 acute iTTP episodes in 41 patients. The TPE cohort comprised 59 acute iTTP episodes in 59 patients.
BMI, body mass index.
One patient from the TPE cohort presented with an acute iTTP relapse, and the diagnosis of iTTP was based on a known history of iTTP, thrombocytopenia, and microangiopathic hemolytic anemia at baseline.
iTTP treatment modalities
The treatment modalities for the TPE-free and TPE cohorts are summarized in Table 2. All patients in this study received caplacizumab as a frontline iTTP treatment. The duration and dose of caplacizumab treatment did not differ significantly between the 2 cohorts. The median duration of caplacizumab treatment was 21.5 and 31 days (interquartile range [IQR], 13-34 and 14.5-47.5) in the TPE-free and TPE cohorts, respectively.
Treatment modalities of the TPE-free and TPE cohorts, treated with frontline caplacizumab
| Parameter . | TPE-free cohort (n = 42) . | TPE cohort (n = 59) . | P value . |
|---|---|---|---|
| Caplacizumab treatment | |||
| Median days of caplacizumab treatment (IQR) | 21.5 (13-34) | 31 (14.5-47.5) | .09 |
| Median total doses of caplacizumab (IQR)∗ | 20 (13-30) | 29 (13-41.5) | .05 |
| Immunosuppression | |||
| Patients treated with steroids, n (%) | 42 (100) | 59 (100) | >.99 |
| Patients treated with rituximab, n (%) | 38 (90.5) | 48 (81.4) | .26 |
| Frontline <72 h, n (%) | 18 (42.9) | 14 (23.7) | .12 |
| Additional immunosuppression during first 30 d, n (%) | 1 (2.4) | 6 (10.2) | .23 |
| Azathioprine | 0 (0) | 3 (5.1) | |
| Bortezomib | 0 (0) | 1 (1.7) | |
| Ciclosporin | 0 (0) | 2 (3.4) | |
| Mycophenolate mofetil | 0 (0) | 0 (0) | |
| Obinutuzumab | 1 (2.4) | 0 (0) | |
| TPE† | |||
| Patients receiving TPE, n (%) | 4 (9.5) | 59 (100) | |
| Median start day of TPE after caplacizumab start (range) | 1.5 (1-2) | 0 (0-1) | |
| Median days of plasma exchange (IQR) | 4.5 (3.5-9) | 5 (3-6) |
| Parameter . | TPE-free cohort (n = 42) . | TPE cohort (n = 59) . | P value . |
|---|---|---|---|
| Caplacizumab treatment | |||
| Median days of caplacizumab treatment (IQR) | 21.5 (13-34) | 31 (14.5-47.5) | .09 |
| Median total doses of caplacizumab (IQR)∗ | 20 (13-30) | 29 (13-41.5) | .05 |
| Immunosuppression | |||
| Patients treated with steroids, n (%) | 42 (100) | 59 (100) | >.99 |
| Patients treated with rituximab, n (%) | 38 (90.5) | 48 (81.4) | .26 |
| Frontline <72 h, n (%) | 18 (42.9) | 14 (23.7) | .12 |
| Additional immunosuppression during first 30 d, n (%) | 1 (2.4) | 6 (10.2) | .23 |
| Azathioprine | 0 (0) | 3 (5.1) | |
| Bortezomib | 0 (0) | 1 (1.7) | |
| Ciclosporin | 0 (0) | 2 (3.4) | |
| Mycophenolate mofetil | 0 (0) | 0 (0) | |
| Obinutuzumab | 1 (2.4) | 0 (0) | |
| TPE† | |||
| Patients receiving TPE, n (%) | 4 (9.5) | 59 (100) | |
| Median start day of TPE after caplacizumab start (range) | 1.5 (1-2) | 0 (0-1) | |
| Median days of plasma exchange (IQR) | 4.5 (3.5-9) | 5 (3-6) |
All parameters are presented per episode. The TPE parameters were not evaluated for statistical significance. The TPE-free cohort comprised 42 acute iTTP episodes in 41 patients. The TPE cohort comprised 59 acute iTTP episodes in 59 patients.
Differences between total days of caplacizumab treatment and total doses of caplacizumab derive from application of alternate-day dosing regimen, as reported by our group.16 Alternate-day dosing was implemented in some patients in both cohorts and did not influence patient outcomes.
In the TPE-free cohort, application of TPE is reported for patients without an adequate response to the TPE-free treatment approach.
TPE in the TPE cohort was initiated on the same day as caplacizumab, except for 1 patient in whom TPE was started with a 1-day delay without explicitly pursuing a TPE-free approach. Daily TPE was performed for a median of 5 days (IQR, 3-6) in the TPE cohort.
All patients received steroids. The use of rituximab for immunosuppression, including frontline use of rituximab within 72 hours, was not significantly different between the cohorts. In the TPE-free cohort, 38 patients (90.5%) received treatment with rituximab, compared with 48 patients (81.4%) in the TPE cohort.
Additional immunosuppressants were administered during the first 30 days to 1 patient in the TPE-free cohort (2.4%) and 6 patients in the TPE cohort (10.2%).
Primary outcome
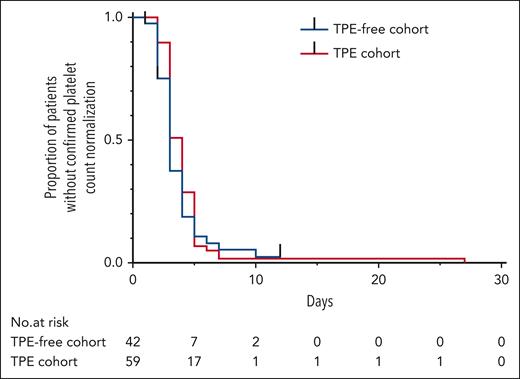
The outcome parameters for the TPE-free and TPE cohorts are summarized in Table 3. The primary outcome parameter, time to platelet count normalization, was not significantly different between TPE-free and TPE-based management (Kaplan-Meier estimator and log-rank test, Table 3; Figure 2). The median time to platelet count normalization was 3 days (IQR, 2-4 days) in the TPE-free cohort and 4 days (IQR, 3-5) in the TPE cohort.
Outcome parameters and safety analysis of the TPE-free and TPE cohorts treated with frontline caplacizumab
| Parameter . | TPE-free cohort (n = 42) . | TPE cohort (n = 59) . | P value . |
|---|---|---|---|
| Primary outcome | |||
| Median time to platelet count normalization (range; IQR), d | 3 (1-12; 2-4) | 4 (2-27; 3-5) | .31 |
| Key secondary outcomes | |||
| Patients achieving a clinical response without requiring subsequent TPE in the TPE-free cohort, n (%) | 38 (90.5) | 57 (96.6) | .23 |
| Patients achieving a clinical response (including patients with subsequent TPE in the TPE-free cohort), n (%) | 41 (97.6) | 57 (96.6) | >.99 |
| Patients with a clinical exacerbation, n (%) | 2 (4.8) | 9 (15.3) | .12 |
| Patients refractory to therapy, n (%) | 0 (0) | 1 (1.7) | >.99 |
| TTP-related death, n (%) | 0 (0) | 1 (1.7) | >.99 |
| Median time to platelet count doubling (range; IQR) | 1 (1-7; 1-2) | 1 (1-4; 1-2) | .88 |
| Other secondary outcomes | |||
| Median time to recovery of ADAMTS13 activity to ≥20% after treatment initiation (IQR), d | 25 (13-33) | 37 (19-51) | .01 |
| Patients without confirmed recovery of ADAMTS13 activity to ≥20% at end of follow-up, n (%) | 4 (9.5) | 10 (16.9) | .39 |
| Median days in hospital (IQR) | 11 (5-15) | 14 (9-21) | .02 |
| Patients admitted to ICU, n (%) | 11 (26.2) | 42 (71.2) | <.01 |
| Data missing, n (%) | 5 (11.9) | 8 (13.6) | |
| Median days on ICU, if admitted (IQR) | 3 (2.5-4.5) | 4 (2-6) | .53 |
| Safety | |||
| Patients with at least 1 reported complication during overall follow-up period, n (%) | 11 (26.2) | 16 (27.1) | >.99 |
| Reported complications on TPE, n (%) | 0 (0) | 4 (6.7) | .14 |
| Any bleeding, n (%) | 5 (11.9) | 2 (3.4) | .12 |
| Major bleeding according to ISTH, n (%) | 2 (4.8) | 0 (0) | .17 |
| Parameter . | TPE-free cohort (n = 42) . | TPE cohort (n = 59) . | P value . |
|---|---|---|---|
| Primary outcome | |||
| Median time to platelet count normalization (range; IQR), d | 3 (1-12; 2-4) | 4 (2-27; 3-5) | .31 |
| Key secondary outcomes | |||
| Patients achieving a clinical response without requiring subsequent TPE in the TPE-free cohort, n (%) | 38 (90.5) | 57 (96.6) | .23 |
| Patients achieving a clinical response (including patients with subsequent TPE in the TPE-free cohort), n (%) | 41 (97.6) | 57 (96.6) | >.99 |
| Patients with a clinical exacerbation, n (%) | 2 (4.8) | 9 (15.3) | .12 |
| Patients refractory to therapy, n (%) | 0 (0) | 1 (1.7) | >.99 |
| TTP-related death, n (%) | 0 (0) | 1 (1.7) | >.99 |
| Median time to platelet count doubling (range; IQR) | 1 (1-7; 1-2) | 1 (1-4; 1-2) | .88 |
| Other secondary outcomes | |||
| Median time to recovery of ADAMTS13 activity to ≥20% after treatment initiation (IQR), d | 25 (13-33) | 37 (19-51) | .01 |
| Patients without confirmed recovery of ADAMTS13 activity to ≥20% at end of follow-up, n (%) | 4 (9.5) | 10 (16.9) | .39 |
| Median days in hospital (IQR) | 11 (5-15) | 14 (9-21) | .02 |
| Patients admitted to ICU, n (%) | 11 (26.2) | 42 (71.2) | <.01 |
| Data missing, n (%) | 5 (11.9) | 8 (13.6) | |
| Median days on ICU, if admitted (IQR) | 3 (2.5-4.5) | 4 (2-6) | .53 |
| Safety | |||
| Patients with at least 1 reported complication during overall follow-up period, n (%) | 11 (26.2) | 16 (27.1) | >.99 |
| Reported complications on TPE, n (%) | 0 (0) | 4 (6.7) | .14 |
| Any bleeding, n (%) | 5 (11.9) | 2 (3.4) | .12 |
| Major bleeding according to ISTH, n (%) | 2 (4.8) | 0 (0) | .17 |
All parameters are presented per episode. The TPE-free cohort comprised 42 acute iTTP episodes in 41 patients. The TPE cohort comprised 59 acute iTTP episodes in 59 patients.
ISTH, International Society on Thrombosis and Haemostasis.
Time to platelet count normalization after the first caplacizumab administration. Symbols indicate censored data. P = .31 for time to platelet count normalization according to the log-rank test.
Time to platelet count normalization after the first caplacizumab administration. Symbols indicate censored data. P = .31 for time to platelet count normalization according to the log-rank test.
Key secondary outcomes
A clinical response without TPE was achieved in 38 patients (90.5%) in the TPE-free group, compared with 57 patients (96.6%) in the TPE group. When patients who subsequently underwent TPE were included, a total of 41 patients (97.6%) achieved a clinical response in the TPE-free cohort.
Clinical exacerbations occurred in 2 patients in the TPE-free cohort (4.8%) and in 9 patients in the TPE cohort (15.3%). Exacerbations in the TPE-free cohort were linked to concomitant cytomegalovirus infection in 1 patient and early termination of caplacizumab in a second patient before ADAMTS13 remission was achieved. In the TPE cohort, all iTTP exacerbations could be attributed to premature termination of caplacizumab treatment, when ADAMTS13 activity was still <10%. Refractory iTTP was not observed in the TPE-free cohort. In the TPE group, refractory TTP was observed in 1 patient (1.7%), who had severe neurological involvement and concomitant aspiration pneumonia. There was 1 TTP-related death reported in the TPE group (1.7%). The cause of death in this patient was cerebral ischemia, which was judged to be unrelated to iTTP treatment.
Short-term treatment response and subsequent initiation of TPE
For evaluation of the short-term treatment response, both cohorts were assessed for the time to platelet count doubling. There was no significant difference in the time to platelet count doubling between the 2 cohorts in the Kaplan-Meier estimator and the log-rank test (Table 3; supplemental Figure 1). The median time to platelet count doubling was 1 day in both cohorts.
Four patients in the TPE-free cohort, in which a TPE-free approach was explicitly pursued by the responsible medical team, did not immediately respond to the first caplacizumab injection based on platelet count levels and subsequently underwent TPE. Details of the clinical courses and treatment modalities of these patients are available in the supplemental Appendix and supplemental Figure 2. The clinical condition of these patients remained stable after the first IV dose of caplacizumab, with no signs of worsening organ function parameters or neurological conditions. The main criterion to add TPE in these patients was a missing increase or even a drop in platelet count. The monitoring period until the addition of TPE to these patients was individual and determined by the responsible medical team.
Three of these patients failed to achieve a platelet count doubling after a maximum monitoring period of nearly 40 hours. A fourth patient achieved platelet count doubling without TPE within 24 hours, but displayed a subsequent drop in platelet count and underwent TPE. In these 4 patients, TPE was initiated at a median of 1.5 days after the first caplacizumab dose (range, 1-2), and TPE was performed for a median of 4.5 days (IQR, 3.5-9). This delayed platelet count response could be attributed to concomitant diseases and factors, such as active human immunodeficiency virus and hepatitis B virus coinfection (supplemental Figure 2A), active cytomegalovirus infection (supplemental Figure 2B), concomitant antiplatelet antibodies in association with an ovarian teratoma (supplemental Figure 2C), and multiple platelet transfusions before the correct diagnosis of TTP has been made (supplemental Figure 2D). The patient with antiplatelet antibodies and a concomitant diagnosis of ovarian teratoma did not achieve normalization of the platelet count during follow-up, despite ADAMTS13 remission and normalization of LDH values. None of the patients met any of the criteria for refractoriness.
A fifth patient from the TPE-free cohort, who showed a robust clinical response to caplacizumab, experienced a decrease in platelet count after 11 days of treatment in association with acute gastrointestinal bleeding (see also the section on safety). At the time of the bleeding event, the LDH level had already normalized. In this patient, caplacizumab was temporarily paused, and TPE was initiated as an alternative iTTP treatment. A detailed case description with clinical courses and treatment modalities is available in the supplemental Appendix.
In the TPE cohort, there were 3 patients with concomitant active diseases that may have impaired the platelet count response, namely the diagnosis of pancreatic cancer, pneumogenic sepsis, and aspiration pneumonia. Despite frontline treatment with caplacizumab and TPE, these patients displayed a prolonged time to platelet count normalization (median, 7 days; range, 7-27 days).
Other secondary outcomes
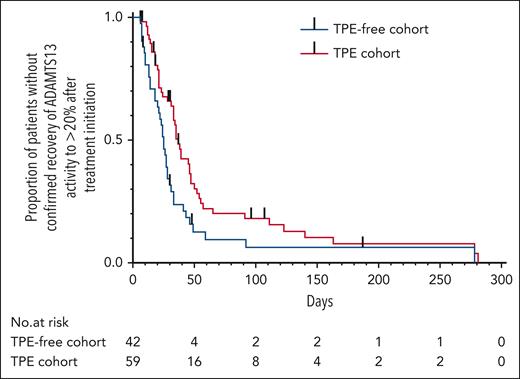
Based on the Kaplan-Meier analysis and a log-rank test, the reported time to recovery of ADAMTS13 activity to ≥20% after treatment initiation was shorter in the TPE-free cohort (Table 3; Figure 3). The median reported time to recovery of ADAMTS13 activity to ≥ 20% after treatment initiation was 25 and 37 days (IQR, 13-33 and 19-51) in the TPE-free and TPE cohorts, respectively.
Time to recovery of ADAMTS13 activity to ≥20% after treatment initiation. Black vertical bars indicate censored data. P = .01 according to the log-rank test.
Time to recovery of ADAMTS13 activity to ≥20% after treatment initiation. Black vertical bars indicate censored data. P = .01 according to the log-rank test.
The duration of hospital stay was significantly shorter in the TPE-free cohort, and fewer patients were admitted to the ICU. The median duration of hospital stay was 11 days in the TPE-free cohort and 14 days in the TPE cohort (IQR, 5-15 and 9-21; P < .02). In the TPE-free cohort, 11 patients (26.2%) were admitted to the ICU, compared with 42 patients (71.2%) in the TPE cohort (P < .001). The duration of care in an ICU per iTTP episode was comparable between the cohorts.
The median time to LDH normalization was 11 days in the TPE-free cohort (supplemental Figure 3).
Safety
During overall follow-up, complications were observed in 11 patients (26.2%) in the TPE-free group and 16 patients (27.1%) in the TPE group, excluding complications of iTTP that were evaluated as outcome parameters (supplemental Table 2).
Serious complications occurred in 4 patients (6.8%) from the TPE cohort while on TPE. These included severe systemic anaphylactic reactions with severe hypotension in 3 patients and generalized seizures and supraventricular tachycardia in a fourth patient. In 1 patient, a systemic anaphylactic reaction led to hemodynamically relevant hypotension and subsequent in-hospital cardiac arrest, with necessity for temporary cardiopulmonary resuscitation.
Bleeding complications were reported without a significant difference between groups and mainly comprised gingival bleeding and epistaxis. Two patients in the TPE-free cohort (4.8%) experienced major bleeding events: a subdural hematoma requiring neurosurgical intervention and gastrointestinal bleeding with mass transfusion. The spontaneous subdural hematoma had occurred in 1 patient after 20 days of caplacizumab treatment and was managed with neurosurgical trepanation and drainage. Gastrointestinal bleeding from a colon diverticulum occurred in another patient after a clinical response to iTTP and resulted in a significant drop in hemoglobin and platelet counts. Gastrointestinal bleeding, to which mild factor XIII deficiency may have contributed, was treated with red blood cell transfusions and a factor XIII concentrate. Details of these bleeding events are described in the supplemental Appendix.
Stratified log-rank test
A stratified log-rank test based on LDH levels was performed to rule out an effect of LDH on the primary outcome (supplemental Figure 4). The primary outcome parameter (time to platelet count normalization) was confirmed in this analysis.
Discussion
In addition to its impact on clinical management and patient outcomes, caplacizumab has paved the way for the management of iTTP without TPE in a growing number of cases.6-9 Here, we present, to our knowledge, the first comprehensive analysis of the largest real-world cohort of patients with acute iTTP managed using a treatment approach aimed at monitoring the initial response to anti-VWF treatment with caplacizumab and omitting TPE if clinically not required. A detailed comparison of this cohort with a control group of iTTP patients treated with frontline caplacizumab, TPE, and immunosuppression allows for an extensive investigation of the role of caplacizumab and TPE in acute iTTP management. For the first time since the introduction of TPE in the early 1990s, the results of our study systematically challenge the absolute need for TPE and suggest a paradigm shift in acute iTTP management that goes beyond individual case reports.10
In detail, our TPE-free treatment approach was successfully implemented in 38 of 42 acute iTTP episodes (90.5%). We observed no significant difference in the time to platelet count normalization between patients with iTTP treated with and without TPE. The analysis of key secondary outcomes did not reveal significant differences in the proportion of patients who achieved a clinical response or experienced exacerbations, refractoriness, or TTP-related deaths. Failure to achieve short-term adequate response in laboratory features of thrombotic microangiopathy and the necessity to start TPE occurred in 4 patients with concomitant diseases and conditions, which may have substantially impaired the immediate platelet count recovery in response to caplacizumab. The main factor for the decision to add TPE in these 4 patients was a lack of platelet count increase. Clinical conditions or neurological status did not deteriorate.
Selection bias due to the tendency to recruit patients with milder iTTP symptoms for TPE-free management may be present in our study. Although there was a significantly higher LDH level at baseline in the TPE cohort, suggesting more severe TTP, parameters of organ damage and the French Severity Score, which can be used to identify patients at risk for adverse outcomes, were not different between the 2 cohorts.21 Of note, neurological involvement was present in more than half of the patients in the TPE-free cohort (57.1%). In addition, differences in initial LDH levels did not show any effect on the primary outcome, based on our stratified analysis.
Despite potential limitations arising from the retrospective design and possible selection bias, the key findings from our study suggest that caplacizumab without TPE is efficacious in controlling thrombotic microangiopathy and achieving a clinical response in acute TTP. The effect of caplacizumab and immunosuppression on the investigated outcome parameters appears to be independent of additional TPE. Evidence from our study is compelling for mild-to-moderately affected patients with TTP, with only limited data on severe cases. However, to date, there is no and with the utmost certainty there will never be evidence for severely affected patients with iTTP beyond retrospective analyses.
In addition to the short-term control of microvascular thrombosis and subsequent organ damage, the modified treatment regimen was efficacious in achieving ADAMTS13 remission, which implies clinical remission and allows for the cessation of anti-VWF medication with caplacizumab. Our data could not confirm the findings of a prolonged time to ADAMTS13 recovery associated with the addition of caplacizumab to TPE, which has been found in a subset of patients with iTTP in the UK TTP registry.22 In line with our data, a recently published analysis of the Spanish TTP registry found no differences in time to ADAMTS13 restoration from TPE start between caplacizumab-treated and non–caplacizumab-treated episodes.23 With a median of 25 days, the reported time to recovery of ADAMTS13 activity to ≥20% after treatment initiation in the TPE-free cohort was well within the range of previously published cohorts.15,22-24 The boost in autoimmunity by TPE has been previously reported and may be a potential cause for the faster recovery we observed in our TPE-free cohort.13 However, despite regular measurements, significant differences between the TPE-free and TPE cohorts in our study may also have resulted from the lack of predefined time points for ADAMTS13 testing. Before systematically generated prospective data becomes available, the influence of caplacizumab or the omission of TPE on the time to ADAMTS13 recovery should be interpreted with caution.
In this retrospective study, there was no significant safety signal favoring 1 treatment option. The number of patients experiencing critical complications was comparable between groups. Although 4 patients experienced serious complications on TPE, including 3 severe systemic anaphylactic reactions (leading to hospital cardiac arrest in 1 case), critical events in the TPE-free cohort comprised 2 major bleeding episodes. However, iTTP treatment with TPE may lead to a prolonged hospital stay, including multiple sessions of daily TPE and the associated costs of plasma, equipment, nursing care, and medical supervision. Besides these procedure-related complications, clinical exacerbations were observed in 4.8% of patients in the TPE-free cohort and 15.3% of patients in the TPE cohort. In all instances except 1, these exacerbations were attributed to the early termination of caplacizumab when ADAMTS13 activity remained below 10%. This finding highlights once again that an ADAMTS13-guided approach is key for the successful management of acute iTTP with caplacizumab.15
Important prerequisites for the implementation of TPE-free patient management may include a high clinical likelihood of TTP when facing thrombotic microangiopathy, fast turn around times for ADAMTS13 testing (preferentially within hours), and on-site availability of caplacizumab (within minutes from the emergency pharmacy). In addition, efficient communication and coordination of involved clinical services (including hematology, nephrology, emergency medicine, intensive care medicine, transfusion medicine, laboratory, pharmacy) are crucial to ensuring prompt collaboration and decision-making. Patient monitoring after caplacizumab administration should include short-term checks of CBC and continuous monitoring for additional TPE.
Based on our analyses, we designed guidance for TPE-free patient management that addresses critical decision points, including the potential need to subsequently initiate TPE (supplemental Figure 5). This guidance closely follows the protocol of the currently ongoing phase 3 open-label, single-arm, multicenter MAYARI trial (ClinicalTrials.gov identifier: NCT05468320), which investigates the efficacy and safety of caplacizumab and immunosuppression without first-line TPE. The results of this trial are expected to complement our research findings and help to further refine treatment protocols for managing iTTP without TPE.
In conclusion, our study indicates that caplacizumab in conjunction with immunosuppression, but without additional TPE, is an effective treatment strategy for the resolution of acute iTTP. Withholding TPE offers several advantages, including simplified treatment protocols, a shorter time to treatment onset, reduced resource burden, and potentially improved patient convenience and safety. Recombinant ADAMTS13, once it has been shown to be effective in iTTP, may further improve outcomes in patients with iTTP.25 Until then, the data presented here provide a basis for TPE-free iTTP management in experienced centers in shared decision-making between patients and treating physicians.
Acknowledgment
The authors thank all participants for their collaboration.
Authorship
Contribution: P.K. and P.T.B. initiated the study; L.K., P.K., L.A.V., and P.T.B. conceptualized the study; L.K., P.K., L.A.V., and P.T.B. wrote, reviewed, and edited the manuscript; L.K., T.O., and L.A.V. calculated statistics; L.K. and L.A.V. prepared the figures; all authors were involved in TPE-free patient management, data collection, and proofreading; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: L.K. received consulting fees from Alexion and research funding from Sanofi-Genzyme. P.K. received consultancy and advisory board fees, speaker honoraria, and travel grants from Ablynx/Sanofi, Alexion, CLS Behring, Novo-Nordisk, Roche, and Shire/Takeda. J.K. reports speaker honoraria and participation on advisory boards from Alexion, Sanofi, and Chiesi. J.M. received speaker honoraria and consultant fees from Ablynx, Alexion, and Sanofi-Genzyme. A.G. reports consulting fees from Sanofi-Genzyme and Alexion and participation in advisory boards for Alexion. D.A.E. has received speaker honoraria from Sanofi-Genzyme and Takeda. L.A.V. received research funding and consulting fees from Alexion, AstraZeneca, Bayer, and Sanofi-Genzyme. P.T.B. received speaker honoraria and consultant fees from AstraZeneca, Alexion, Bayer, Novartis, Roche, Sanofi-Genzyme, Travere, Vifor CSL and participated in advisory boards for Alexion, Sanofi-Genzyme, Novartis, Travere, Takeda, Vifor CSL, and Bayer, and declares research funding from the German Research Foundation BR-2955/8 and Sanofi-Genzyme. The remaning authors declare no competing financial interests.
A complete list of the members of the Austrian Thrombotic Microangiopathy Registry and the REACT-2020 TTP Study Group who contributed cases to this study, appears in the supplemental Appendix.
Correspondence: Paul Knöbl, Division of Hematology and Hemostasis, Department of Medicine 1, Medical University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria; email: paul.knoebl@meduniwien.ac.at; and Paul T. Brinkkoetter, University of Cologne, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, Kerpener Str 62, D-50937 Cologne, Germany; email: paul.brinkkoetter@uk-koeln.de.
References
Author notes
L.K. and P.K. contributed equally to this study.
L.A.V. and P.T.B. are joint senior authors.
Individual participant data will not be made available due to the requirements of the local ethics review boards and data protection regulations. The statistical analysis plan and data will be shared with investigators who provide a methodologically sound proposal. Proposals should be directed to the corresponding authors, Paul Knöbl (paul.knoebl@meduniwien.ac.at) or Paul T. Brinkkoetter (paul.brinkkoetter@uk-koeln.de). To gain access, data requestors will need to sign a data access agreement.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

Comments
Potential inclusion of milder iTTP cases in the TPE-Free Cohort
The TPE-free cohort included patients with immediate platelet recovery after the first caplacizumab dose and stable clinical conditions. Additionally, lactate dehydrogenase levels at onset and ICU admission rates were significantly lower in the TPE-free cohort, suggesting the cohort may include milder cases.
Furthermore, the levels of ADAMTS13 inhibitor or anti-ADAMTS13 IgG either at onset or during the treatment course were unclear. As the persistence of ADAMTS13 inhibitor may lead to delayed recovery of ADAMTS13 activity1, ADAMTS13 inhibitor levels should be measured during the disease course. Additionally, based on the residual ADAMTS13 inhibitor levels, antibody removal through TPE or further immunosuppression may be required for the early recovery of ADAMTS13 activity, which may help prevent the prolonged use of caplacizumab.
1Saito K et al (2024) Blood Adv, 8, 2151-2159
Conflict-of-interest disclosure
K.Sakai received lecture fees from Sanofi. M.M. provided consultancy services for Takeda, Alexion Pharma, and Sanofi; received speaker fees for Takeda, Alexion Pharma, Asahi Kasei Pharma, and Sanofi; and received research funding from Alexion Pharma, Chugai Pharmaceutical, Asahi Kasei Pharma, and Sanofi. K.Saito declares no competing financial interests.