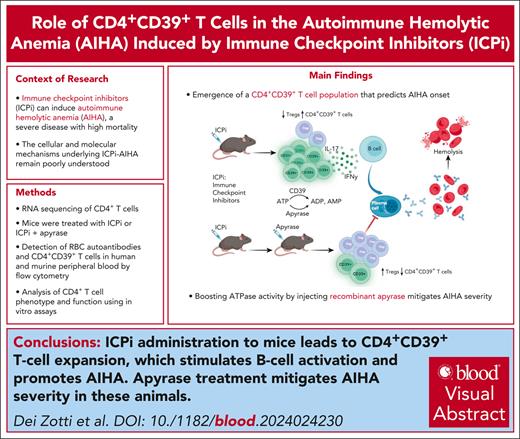
Key Points
Discovery of a novel CD39+ T-cell population that predicts AIHA onset due to ICPi therapy.
This study demonstrates that apyrase acts as a novel therapeutic, effectively mitigating ICPi-induced AIHA.
Visual Abstract
Immune checkpoint inhibitors (ICPis) have revolutionized cancer immunotherapy but also can induce autoimmune hemolytic anemia (AIHA), a severe disease with high mortality. However, the cellular and molecular mechanism(s) of AIHA secondary to ICPi therapy (ICPi-AIHA) are unclear, other than being initiated through decreased checkpoint inhibition. Herein, we report ICPi-AIHA in a novel mouse model that shows similar characteristics of known human ICPi-AIHA (eg, autoantibodies, hemolysis, and increased mortality). During ICPi-AIHA, there is the simultaneous reduction of 2 regulatory T-cell populations (FoxP3+ and Tr1 [type 1 regulatory cells]) and an increase in inflammatory T helper cell 17 (TH17). Moreover, a novel CD39+CD73–FoxP3–CD25– CD4+ T-cell subset (ie, CD39 single positive [CD39SP]) emerges, and early increases in CD39SP predict AIHA development; CD39 is an ectonuclease that breaks down adenosine triphosphate (ATP). Additionally, we found that boosting ATPase activity by injecting recombinant apyrase mitigates AIHA development and significant CD39SP reductions, both suggesting a functional role for CD39 and demonstrating a novel therapeutic approach. Importantly, CD39SP are detectable in multiple mouse models developing AIHA and in patients with AIHA, demonstrating applicability to idiopathic and secondary AIHA. Highlighting broader autoimmunity relevance, ICPi-treated NZB mice experienced accelerated onset and severity of lupus, including AIHA. Moreover, ICPi treatment of healthy B6 animals led to detectable CD39SP and development of autoantibodies against multiple autoantigens including those on red blood cells and platelets. Together, our findings provide further insight into the cellular and molecular mechanisms of ICPi-AIHA, leading to novel diagnostic and therapeutic approaches with translational potential for use in humans being treated with ICPi.
Introduction
Immune checkpoint inhibitors (ICPis) have redefined the landscape of cancer immunotherapy by blocking pathways that dampen immune responses to tumor antigens.1-3 Because these pathways mediate immune tolerance, ICPis also induce autoimmune pathologies in some patients.4 Immune-related adverse events (irAEs) can affect multiple organs and systems. The most frequently reported irAEs in the hematologic compartment is the induction of autoimmune hemolytic anemia (AIHA) that can result in severe hemolysis and high mortality rates.5-7 Although the cause of irAEs seems obvious (ie, checkpoint inhibition), the underlying immunological mechanisms of AIHA secondary to ICPi therapy (ICPi-AIHA) has been largely unexplored.
In studies with red blood cell (RBC) transgenic mice, we previously reported that T-cell tolerance is required to prevent AIHA.8 In addition, unlike most autoantigens, tolerization of RBC autoreactive T cells does not occur in the thymus; instead, autoreactive CD4 recent thymic emigrants (RTEs) encounter RBCs in the periphery and upregulate the expression of inhibitory receptors (eg, programmed cell death protein 1 [PD1]) along with transcription factors associated with anergy, exhaustion, and regulatory T cells (Tregs).9 Although these events together produce stringent tolerance to RBC autoantigens, they are frequently abrogated in females and upon aging, as seen in human patients presenting with AIHA.10,11
These studies developed a new model of ICPi-AIHA. We demonstrate that ICPis targeting the specific pathways associated with RTE tolerance to RBC autoantigens caused decreases in 2 Treg populations (FoxP3+ and Tr1) and expansion of proinflammatory TH17 T cells. Simultaneously, a novel CD4+ T-cell subset that expresses CD39 (ie, CD39 single positive [CD39SP]) emerged; the presence of CD39SP predicted subsequent AIHA development in ICPi-treated mice. This model of ICPi-induced tolerance failure to RBC autoantigens, accompanied by the appearance of CD39SP, also occurred in both lupus-prone NZB (New Zealand Black) mice and healthy wild-type B6 mice. Finally, because CD39 functions as an adenosine triphosphate (ATP) hydrolase, injection of apyrase to supplement CD39’s enzymatic activity (ie, ectonuclease) mitigated ICPi-AIHA.
Together, the findings establish a causal role for specific immunoregulatory molecules in RBC autoantigen tolerance, generate a novel model for a major sequela of ICPi therapy, identify a new subset of T cells that predicts AIHA development, and introduce a novel therapeutic intervention. Although each of these findings were generated in murine models, we report the novel finding that humans with AIHA have increased CD39SP compared with healthy controls, supporting the potential translatability of these findings.
Materials and methods
Mice
C57BL/6 (B6) (C57/BL6NCr; stock number 556) mice were purchased from Charles River. OTII (B6.Cg-Tg[TcraTcrb]425CBn/J; stock number 004194) and NZB (NZBWF1/J; stock number 100008) mice were purchased from The Jackson Laboratory. HOD mice and HODxOTII and HODxOTII.Rag2p-GFP F1 mice were generated as described.9,10,12 HOD mice express an RBC-specific triple fusion protein consisting of hen egg lysozyme (HEL), ovalbumin (OVA), and human blood group molecule Duffy. To control for unknown background genetic effects, breeding was performed with heterozygous HOD mice, and HOD+OTII+ and HODNEGOTII+ mice were littermates. Mice were maintained in a light- and temperature-controlled, pathogen-free environment on standard chow and water and phenotyped by flow cytometry. Protocols were approved by the Columbia University Institutional Animal Care and Use Committee.
Human peripheral blood samples
Anonymized residual whole blood samples from individuals diagnosed with AIHA were collected in EDTA and received from Columbia University’s Center for Advanced Laboratory Medicine through an honest broker protocol approved by the Columbia University Institutional Review Board. Samples were deidentified, and only age, sex, and ICD-10 (International Classification of Diseases, 10th Revision) codes were collected (Table 1). AIHA diagnosis was established through laboratory evaluation. Peripheral blood mononuclear cells (PBMCs) were isolated as described.13
Demographic characteristics, number of CD4+T cells in 1 mL of blood collected from patients with AIHA and healthy volunteers
| Sample ID . | Sex . | Age, y . | Number of CD4+× 103/L . | % of FoxP3–CD39+/CD4+ . | Hgb . | Indirect bilirubin . | LDH . | Haptoglobin . | History . | Treatment . |
|---|---|---|---|---|---|---|---|---|---|---|
| AIHA-1 | F | 82 | 8 364 | 8.09 | 3.9 | 7.7 | 1396 | <20 | CLL and severe AIHA | High-dose steroids, IVIG, and rituximab |
| AIHA-2 | M | 67 | 16 202 | 3.6 | 6.8 | 2.2 | 352 | <20 | Systemic mastocytosis, cytopenias, and Evans syndrome | High-dose steroids, IVIG, and rituximab |
| AIHA-3 | M | 59 | 16 855 | 5.29 | 3.9 | 3.9 | 2801 | <10 | Diabetes type 2, severe AIHA mixed warm and cold autoantibodies | High-dose steroids, plasma exchange, and rituximab |
| AIHA-4 | M | 76 | 3 141 | 31.5 | 6.6 | 2.3 | 350 | <20 | Follicular lymphoma, hemolysis, and warm autoantibodies | Rituximab |
| AIHA-5 | M | 71 | 11 056 | 10.4 | 3.1 | 2.7 | 953 | <20 | CLL and AIHA | High-dose steroids |
| AIHA-6 | M | 58 | 5 123 | 30.4 | 5.8 | 0.7 | 665 | <20 | AITL and AIHA | High-dose steroids, etoposide, and belinostat for AITL |
| AIHA-7 | M | 4 | 14 612 | 12.6 | 5.2 | 1 | 710 | Dilated cardiomyopathy, pure red cell aplasia, and AIHA | High-dose steroids and rituximab | |
| AIHA-8 | M | 84 | 9 736 | 10.11 | 8.3 | 1.8 | 669 | <20 | CLL, warm and cold autoantibodies, and severe anemia | High-dose steroids |
| AIHA-9 | M | 21 | 9 805 | 7.15 | 6.3 | 2.1 | 403 | <20 | Evans syndrome, warm AIHA, and CLL | High-dose steroids, IVIG, rituximab, and cytoxan |
| Healthy-1 | M | 37 | 46 116 | 0.21 | ||||||
| Healthy-2 | F | 36 | 46 202 | 3.91 | ||||||
| Healthy-3 | F | 23 | 42 875 | 2.3 | ||||||
| Healthy-4 | F | 27 | 24 138 | 1.59 | ||||||
| Healthy-5 | F | 24 | 45 431 | 2.14 | ||||||
| Healthy-6 | F | 42 | 63 190 | 1.45 | ||||||
| Healthy-7 | F | 62 | 66 387 | 3.56 | ||||||
| Healthy-8 | F | 71 | 47 738 | 4.3 | ||||||
| Healthy-9 | M | 71 | 52 723 | 0.9 | ||||||
| Healthy-10 | M | 55 | 70 446 | 14.9 | ||||||
| Healthy-11 | M | 51 | 110 017 | 3.4 | ||||||
| Healthy-12 | F | 65 | 111 893 | 13 | ||||||
| Healthy-13 | M | 63 | 63 711 | 3.03 | ||||||
| Healthy-14 | M | 50 | 75 011 | 3.65 | ||||||
| Healthy-15 | M | 63 | 57 511 | 7.81 |
| Sample ID . | Sex . | Age, y . | Number of CD4+× 103/L . | % of FoxP3–CD39+/CD4+ . | Hgb . | Indirect bilirubin . | LDH . | Haptoglobin . | History . | Treatment . |
|---|---|---|---|---|---|---|---|---|---|---|
| AIHA-1 | F | 82 | 8 364 | 8.09 | 3.9 | 7.7 | 1396 | <20 | CLL and severe AIHA | High-dose steroids, IVIG, and rituximab |
| AIHA-2 | M | 67 | 16 202 | 3.6 | 6.8 | 2.2 | 352 | <20 | Systemic mastocytosis, cytopenias, and Evans syndrome | High-dose steroids, IVIG, and rituximab |
| AIHA-3 | M | 59 | 16 855 | 5.29 | 3.9 | 3.9 | 2801 | <10 | Diabetes type 2, severe AIHA mixed warm and cold autoantibodies | High-dose steroids, plasma exchange, and rituximab |
| AIHA-4 | M | 76 | 3 141 | 31.5 | 6.6 | 2.3 | 350 | <20 | Follicular lymphoma, hemolysis, and warm autoantibodies | Rituximab |
| AIHA-5 | M | 71 | 11 056 | 10.4 | 3.1 | 2.7 | 953 | <20 | CLL and AIHA | High-dose steroids |
| AIHA-6 | M | 58 | 5 123 | 30.4 | 5.8 | 0.7 | 665 | <20 | AITL and AIHA | High-dose steroids, etoposide, and belinostat for AITL |
| AIHA-7 | M | 4 | 14 612 | 12.6 | 5.2 | 1 | 710 | Dilated cardiomyopathy, pure red cell aplasia, and AIHA | High-dose steroids and rituximab | |
| AIHA-8 | M | 84 | 9 736 | 10.11 | 8.3 | 1.8 | 669 | <20 | CLL, warm and cold autoantibodies, and severe anemia | High-dose steroids |
| AIHA-9 | M | 21 | 9 805 | 7.15 | 6.3 | 2.1 | 403 | <20 | Evans syndrome, warm AIHA, and CLL | High-dose steroids, IVIG, rituximab, and cytoxan |
| Healthy-1 | M | 37 | 46 116 | 0.21 | ||||||
| Healthy-2 | F | 36 | 46 202 | 3.91 | ||||||
| Healthy-3 | F | 23 | 42 875 | 2.3 | ||||||
| Healthy-4 | F | 27 | 24 138 | 1.59 | ||||||
| Healthy-5 | F | 24 | 45 431 | 2.14 | ||||||
| Healthy-6 | F | 42 | 63 190 | 1.45 | ||||||
| Healthy-7 | F | 62 | 66 387 | 3.56 | ||||||
| Healthy-8 | F | 71 | 47 738 | 4.3 | ||||||
| Healthy-9 | M | 71 | 52 723 | 0.9 | ||||||
| Healthy-10 | M | 55 | 70 446 | 14.9 | ||||||
| Healthy-11 | M | 51 | 110 017 | 3.4 | ||||||
| Healthy-12 | F | 65 | 111 893 | 13 | ||||||
| Healthy-13 | M | 63 | 63 711 | 3.03 | ||||||
| Healthy-14 | M | 50 | 75 011 | 3.65 | ||||||
| Healthy-15 | M | 63 | 57 511 | 7.81 |
Frequency of CD39SP cells with phenotype CD4+CD39+CD73–FoxP3–CD25– was calculated. Laboratory evaluation of patients with AIHA. AITL, angioimmunoblastic T-cell lymphoma; CLL, chronic lymphocytic leukemia; F, female; Hgb, hemoglobin; IVIG, IV immunoglobulin; LDH, lactate dehydrogenase; M, male.
RNA sequencing
Total RNA was extracted from sorted splenic CD4+ T cells collected from HODxOTII F1 mice and sent to the Genome Technology Access Center at Washington University (St. Louis, MO) for RNA sequencing.
ICPi cocktail and apyrase injections
Mice were injected intraperitoneally with a 4-antibody cocktail against interleukin-10 receptor (IL-10R), CTLA-4 (cytotoxic T-lymphocyte associated protein 4), Lag-3 (lymphocyte-activation gene 3), and PD1. Apyrase from Pichia pastoris was administered for prophylactic (3 units of apyrase or inactivated apyrase14) or therapeutic administration (5 units).
Peripheral blood analysis
Peripheral blood was assessed for autoantibodies using direct antiglobulin test and flow crossmatch, as described.10 To evaluate anemia, Ter119+CD71high reticulocyte frequency, hematocrit, and hemoglobin were determined as described.15 Reactive oxygen species was detected using CM-H2DCFDA (DCFDA) probe, and CD39SP was detected in whole blood. Serum blood urea nitrogen (BUN) and creatinine were measured by the Clinical Laboratory Improvement Amendments–certified clinical diagnostic laboratory at Columbia University (Roche Cobas). Serum dsDNA autoantibodies, bilirubin, and cytokines were quantified using assay kits, per manufacturer’s instructions.
Kidney pathology
Kidneys harvested from 6-month-old NZB mice were bivalved and fixed in 10% paraformaldehyde for 24 hours. Periodic acid–Schiff staining was performed on 3-μm paraffin-embedded tissue sections. Histologic features of lupus nephritis in glomerular, tubulointerstitial, and vascular compartments were analyzed and scored by a blind assessor using an Olympus BX41 light microscope.
Spleen analysis and flow cytometry
Spleens were processed into single splenocyte suspensions. CD4+ and CD39SP T cells were sorted as described elsewhere16 with a Sony MA900 and cocultured with B cells or stained with antibodies to detect checkpoint molecules, Tregs, purinergic signaling, and naïve, effector, and memory T cells. To assess T helper cells (THs) and Tr1 Tregs, cells were incubated at 37°C with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 500 ng/mL ionomycin for 24 or 72 hours, respectively. In vitro suppression assays were performed using a modified protocol previously described.17
Statistical analysis
Comparison of means between 2 groups was analyzed with the Mann-Whitney test. Experiments with ≥3 groups were analyzed with a 1-way analysis of variance with the Sidak comparisons post test. For longitudinal analysis of ≥3 groups, a repeated measures 2-way analysis of variance with multiple comparisons test was used. Data were analyzed in GraphPad Prism, and P value <.05 was considered significant.
Antibodies and cell processing reagents are provided in supplemental Table 1, available on the Blood website. Please see supplemental Material for additional details on methods.
Results
RBC autoreactive CD4+ T cells tolerization involves checkpoint molecules, Treg development, and purinergic signaling molecules
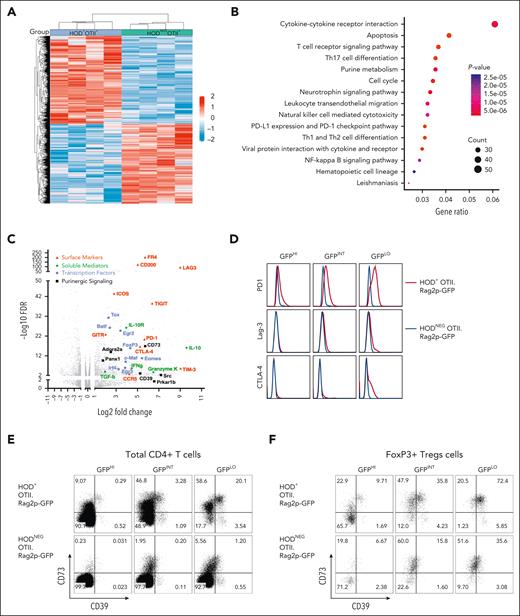
The HOD mouse expresses an RBC-specific surface protein containing the peptide (OVA323-339) recognized by OTII CD4+ T cells. We previously reported that RBC autoreactive CD4+ T cells from HOD+OTII+ are tolerized as RTEs9 and that tolerance fails, leading to AIHA, upon aging (ie, ∼5 months).10 To identify RTE tolerization pathways, RNA sequencing (RNAseq) was performed on sorted CD4+ T cells from young autoreactive HOD+OTII+ and littermate negative control HODNEGOTII+ mice. Hierarchical clustering of transcripts revealed significant differences between tolerized and control CD4+ T cells (Figure 1A). Pathway analysis highlighted significant changes in gene expression in multiple pathways including T-cell receptor signaling, TH17 cell differentiation, and purine metabolism (Figure 1B). Tolerized CD4+ T cells from autoreactive HOD+OTII+ mice significantly upregulated checkpoint molecules (eg, PD1, Lag-3, and CTLA-4),18 immunosuppressive cytokines (eg, IL-10, transforming growth factor β, and IL-10R),19,20 purinergic signaling molecules (eg, CD39 and CD73),21 and Treg-associated transcription factors (eg, FoxP3, Egr2, and Eomes; Figure 1C). This enrichment of transcription factors and soluble mediators was consistent with at least 2 known Treg subsets: FoxP3+ Tregs that secrete immunosuppressive IL-10 and transforming growth factor β, and Tr1 Tregs that can express Eomes, c-MAF, Egr2, and IRF4 and secrete granzyme K and interferon gamma (IFN-γ).22-24
RBC autoreactive RTE tolerization involves checkpoint molecules and Tregs. CD4+ T cells from 10- to 12-week-old HODxOTII F1 mice were sorted for RNAseq. (A) Heat map representing differences of transcript expression in CD4+ T cells from autoreactive HOD+OTII+ mice (blue) and littermate control HODNEGOTII+ mice (green; n = 4 per genotype). (B) Dot plot showing pathway enrichment from differential gene expression analysis. Dot size represents the number of genes in each pathway; red to blue scale of P values indicates red < purple < blue. (C) Plot representing upregulated transcripts in CD4+ T cells from autoreactive HOD+OTII+, compared with littermate control HODNEGOTII+ mice. Transcripts are grouped in colors: those associated with anergy and/or exhaustion (red), immunosuppressive cytokines (green), Tregs (blue), and purinergic signaling (black). Data are combined from 4 mice of each genotype. (D) Representative histograms of checkpoint molecules PD1, Lag-3, and CTLA-4 expression on CD4+ T cells from HOD+OTII.Rag2p-GFP (red) and littermate control HODNEGOTII.Rag2p-GFP (blue) mice. CD4+ T cells from HOD+OTII.Rag2p-GFP and HODNEGOTII.Rag2p-GFP mice were sorted based on GFP expression, an indirect measure of T-cell age: GFPHI (ie, RTEs) on the left, GFPINT in the middle, and GFPLO (ie, mature naïve T cells) on the right. (E-F) Expression of CD73 and CD39 on (E) total CD4+Vα2+Vβ5+ OTII T cells and on (F) CD4+FoxP3+CD25+Vα2+Vβ5+ Tregs from HOD+OTII.Rag2p-GFP (top row) and HODNEGOTII.Rag2p-GFP (bottom row). Data in panels D-E are representative of 3 mice per group and 3 independent experiments.
RBC autoreactive RTE tolerization involves checkpoint molecules and Tregs. CD4+ T cells from 10- to 12-week-old HODxOTII F1 mice were sorted for RNAseq. (A) Heat map representing differences of transcript expression in CD4+ T cells from autoreactive HOD+OTII+ mice (blue) and littermate control HODNEGOTII+ mice (green; n = 4 per genotype). (B) Dot plot showing pathway enrichment from differential gene expression analysis. Dot size represents the number of genes in each pathway; red to blue scale of P values indicates red < purple < blue. (C) Plot representing upregulated transcripts in CD4+ T cells from autoreactive HOD+OTII+, compared with littermate control HODNEGOTII+ mice. Transcripts are grouped in colors: those associated with anergy and/or exhaustion (red), immunosuppressive cytokines (green), Tregs (blue), and purinergic signaling (black). Data are combined from 4 mice of each genotype. (D) Representative histograms of checkpoint molecules PD1, Lag-3, and CTLA-4 expression on CD4+ T cells from HOD+OTII.Rag2p-GFP (red) and littermate control HODNEGOTII.Rag2p-GFP (blue) mice. CD4+ T cells from HOD+OTII.Rag2p-GFP and HODNEGOTII.Rag2p-GFP mice were sorted based on GFP expression, an indirect measure of T-cell age: GFPHI (ie, RTEs) on the left, GFPINT in the middle, and GFPLO (ie, mature naïve T cells) on the right. (E-F) Expression of CD73 and CD39 on (E) total CD4+Vα2+Vβ5+ OTII T cells and on (F) CD4+FoxP3+CD25+Vα2+Vβ5+ Tregs from HOD+OTII.Rag2p-GFP (top row) and HODNEGOTII.Rag2p-GFP (bottom row). Data in panels D-E are representative of 3 mice per group and 3 independent experiments.
To validate the RNAseq data and evaluate protein expression as RTEs become tolerized, CD4+ T cells from HODxOTII.Rag2p-GFP F1 mice were sorted based on GFP intensity, an indirect measure of time since thymic emigration; GFP signal is brightest in RTEs (ie, “GFPHI”) and decays over 3 weeks as T cells mature (ie, “GFPLO”).9 Compared with controls, tolerized CD4+ RTEs from HOD+OTII.Rag2p-GFP mice progressively upregulated PD1, Lag-3, CTLA-4, ICOS, GITR, and CD200 as they matured (Figure 1D; supplemental Figure 1A). Consistent with prior observations,9 low FoxP3+ Treg frequencies were detectable in autoreactive GFPHI RTEs, which expanded upon maturation (Figure 1E; supplemental Figure 1B). Based on our RNAseq data and ATP hydrolysis as a well-described Treg suppression mechanism,20,25 expression of purinergic signaling molecules CD39 and CD73 was interrogated. Compared with controls, there was a significant increase in the frequency of CD73+ cells in autoreactive GFPHI RTEs, which further increased during maturation (Figure 1E; supplemental Figure 1C). Significant frequencies of autoreactive GFPINT and GFPLO RTEs also exhibited detectable coexpression of CD39 and CD73, compared with controls. FoxP3+ Tregs coexpressed CD39 and CD73 (Figure 1F; supplemental Figure 1D). Intriguingly, CD73 upregulation preceded FoxP3+ Treg development, suggesting a role for purinergic signaling in tolerization to RBC autoantigens.
ICPi reverse tolerance to RBC autoantigens and initiate AIHA
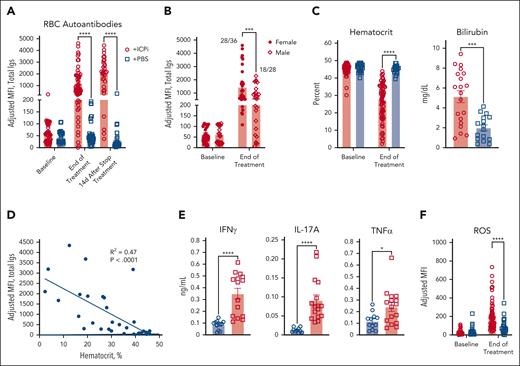
AIHA is a known side effect of ICPi immunotherapy.6,7,26 Considering this observation, together with genes identified by RNAseq, we tested whether tolerance could be reversed in young HOD+OTII+ mice by treating with blocking antibodies against checkpoint molecules PD1, Lag-3, and CTLA-4 as well as the immunosuppressive cytokine IL-10R, denoted as “ICPi.” Within 2 weeks, ICPi-treated HOD+OTII+ mice had high levels of RBC autoantibodies, which continued to increase after treatment cessation (Figure 2A). Similar to humans,27-29 female mice had higher autoantibody levels than males (Figure 2B). Autoantibodies were also detectable on RBCs from ICPi-treated mice but not controls (supplemental Figure 2A). ICPi treatment induced splenomegaly (not shown) and anemia, evidenced by significantly reduced hematocrit and hemoglobin levels, elevated serum bilirubin levels, and reticulocytosis (Figure 2C; supplemental Figure 2B); there was a significant correlation between disease severity and autoantibody levels (Figure 2D). ICPi treatment stimulated production of multiple proinflammatory cytokines, including IFN-γ, IL-17, and tumor necrosis factor α (TNFα) (Figure 2E; supplemental Figure 2C).30 Moreover, RBCs from autoantibody-producing mice exhibited elevated levels of detectable reactive oxygen species, likely influencing AIHA pathogenesis (Figure 2F).31 Together, these data demonstrate that ICPi induces AIHA in our mouse model.
ICPi treatment promotes AIHA development in HOD+OTII+ mice. Young (aged 2-3 months) male and female HOD+OTII+ mice were treated 14 days with antibodies against PD1, Lag-3, CTLA-4, and IL-10R (ie, ICPi, represented in red). Control mice were treated with phosphate-buffered saline (PBS; blue). (A) Whole blood was collected at baseline, after 14 days of treatment, and 14 days after treatment cessation, and sera were analyzed for RBC autoantibodies by flow crossmatch. (B) The frequency of autoantibodies after 14 days of ICPi treatment was stratified by sex: 28 of 36 females and 18 of 28 males produced autoantibodies. (C) Hematocrit and bilirubin concentration demonstrates that ICPi treated (red) but not control mice (blue) exhibited RBC hemolysis and became anemic. (D) Linear regression analysis showing a significant negative correlation between RBC autoantibody levels and hematocrit (R2 = 0.47; P < .0001). (E) Serum cytokine levels 2 weeks after ICPi or PBS control treatment. (F) Levels of reactive oxygen species (ROS) in peripheral RBCs. Data in panels A, B, C, and F are combined from 6 independent experiments with 4 mice per group. Data for bilirubin are combined from 3 to 5 independent experiment with 4 mice/group. Data in panel E are combined from 2 independent experiment with 10 mice per group. Statistical significance was determined by Pearson correlation, Mann-Whitney test, or repeated measures 2-way analysis of variance (ANOVA) with Sidak multiple comparisons test: ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Igs, total immunoglobulins; MFI, mean fluorescence intensity.
ICPi treatment promotes AIHA development in HOD+OTII+ mice. Young (aged 2-3 months) male and female HOD+OTII+ mice were treated 14 days with antibodies against PD1, Lag-3, CTLA-4, and IL-10R (ie, ICPi, represented in red). Control mice were treated with phosphate-buffered saline (PBS; blue). (A) Whole blood was collected at baseline, after 14 days of treatment, and 14 days after treatment cessation, and sera were analyzed for RBC autoantibodies by flow crossmatch. (B) The frequency of autoantibodies after 14 days of ICPi treatment was stratified by sex: 28 of 36 females and 18 of 28 males produced autoantibodies. (C) Hematocrit and bilirubin concentration demonstrates that ICPi treated (red) but not control mice (blue) exhibited RBC hemolysis and became anemic. (D) Linear regression analysis showing a significant negative correlation between RBC autoantibody levels and hematocrit (R2 = 0.47; P < .0001). (E) Serum cytokine levels 2 weeks after ICPi or PBS control treatment. (F) Levels of reactive oxygen species (ROS) in peripheral RBCs. Data in panels A, B, C, and F are combined from 6 independent experiments with 4 mice per group. Data for bilirubin are combined from 3 to 5 independent experiment with 4 mice/group. Data in panel E are combined from 2 independent experiment with 10 mice per group. Statistical significance was determined by Pearson correlation, Mann-Whitney test, or repeated measures 2-way analysis of variance (ANOVA) with Sidak multiple comparisons test: ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001. Igs, total immunoglobulins; MFI, mean fluorescence intensity.
RBC tolerance reversal with ICPi is generalizable
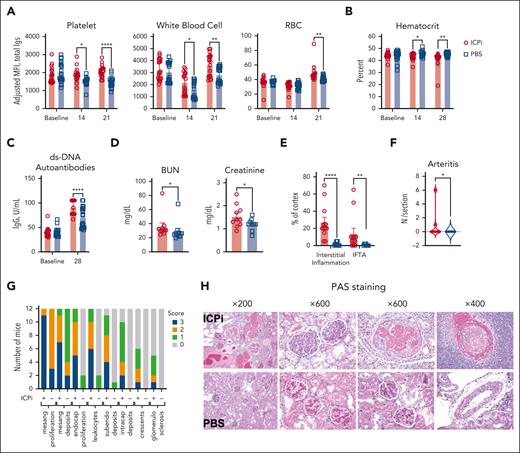
To test whether tolerance failure mechanisms for RBC autoantigens is shared by other widely expressed autoantigens, NZB mice received ICPi; NZB mice are a well-characterized model of spontaneous development of human systemic lupus erythematosus that produces age-onset autoantibodies reactive to antinuclear, platelet, and RBC autoantigens, as well as severe kidney disease at age 6 months.32 Within 2 weeks, ICPi treatment promoted significant increases in detectable autoantibodies bound to peripheral platelets and white blood cells and, by 3 weeks, to RBCs (Figure 3A). ICPi-treated NZB mice had significantly decreased hematocrit (Figure 3B), and increased anti-dsDNA autoantibody levels, a hallmark of lupus progression (Figure 3C). To assess whether ICPi treatment hastened other lupus features, serum and tissue were analyzed 3 months after treatment cessation. BUN and creatinine were significantly increased in ICPi-treated NZB mice (Figure 3D), and periodic acid–Schiff-stained kidney sections showed findings characteristic of severe active and progressive lupus nephritis, whereas control mice showed a mild phenotype (Figure 3E-H). To test whether ICPi could facilitate tolerance failure in otherwise healthy animals, without a genetic predisposition to autoimmunity, B6 mice were treated with ICPi; similar kinetics of autoantibody production and reactivity were observed (supplemental Figure 3). These data show that ICPi treatment accelerates autoimmunity in NZB mice and induces autoantibodies in healthy B6 animals, demonstrating a key role for these pathways in tolerance to multiple autoantigens expressed on various cell subsets and tissues.
ICPi accelerates autoantibody production and lupus erythematosus onset in young NZB mice. NZB mice (6-week-old females) were treated with ICPi (red) or PBS (blue) for 28 days. (A) Autoantibodies bound to peripheral CD41+ platelets, CD45+ white blood cells, and Ter119+ RBCs were assessed before and during ICPi treatment with a direct antiglobulin test by staining with anti-mouse Igs APC. (B) Hematocrit was evaluated before, during (at 14 days), and at the cessation of ICPi treatment (at 28 days). (C-D) Serum was collected to quantify double-stranded DNA (dsDNA) autoantibodies by enzyme-linked immunosorbent assay (ELISA) at ICPi treatment cessation (C) and blood urea nitrogen (BUN) and creatinine at the time of euthanasia (ie, 3 months after treatment cessation) (D). Kidneys from ICPi- and PBS-treated NZB mice were collected, formalin fixed, and paraffin embedded. Histologic findings in periodic acid–Schiff (PAS)-stained coronal kidney sections were analyzed. (E-F) Percent of total cortical parenchyma with interstitial inflammation, interstitial fibrosis/tubular atrophy (IFTA) (E), and the number of arteritis lesions per tissue section (F) was calculated. (G) Glomerular features including mesangial hypercellularity, mesangial deposits, endocapillary hypercellularity, leukocyte infiltration, subendoethlial and intracapillary deposits, crescents, and glomerulosclerosis were graded semiquantitatively on a scale of 0 to 3+ (0, absent; 1+, involving 1%-25% glomeruli; 2+, involving 26%-50% glomeruli; 3+, involving >50% glomeruli per coronal section of kidney containing >150 glomeruli per mouse). (H) Representative histologic PAS-stained kidney sections are shown. Data are cumulative from 2 independent experiments with 10 mice per group. Statistical significance was determined by Mann-Whitney test or repeated measures 2-way ANOVA with Sidak multiple comparisons test: ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. IgG, immunoglobulin G.
ICPi accelerates autoantibody production and lupus erythematosus onset in young NZB mice. NZB mice (6-week-old females) were treated with ICPi (red) or PBS (blue) for 28 days. (A) Autoantibodies bound to peripheral CD41+ platelets, CD45+ white blood cells, and Ter119+ RBCs were assessed before and during ICPi treatment with a direct antiglobulin test by staining with anti-mouse Igs APC. (B) Hematocrit was evaluated before, during (at 14 days), and at the cessation of ICPi treatment (at 28 days). (C-D) Serum was collected to quantify double-stranded DNA (dsDNA) autoantibodies by enzyme-linked immunosorbent assay (ELISA) at ICPi treatment cessation (C) and blood urea nitrogen (BUN) and creatinine at the time of euthanasia (ie, 3 months after treatment cessation) (D). Kidneys from ICPi- and PBS-treated NZB mice were collected, formalin fixed, and paraffin embedded. Histologic findings in periodic acid–Schiff (PAS)-stained coronal kidney sections were analyzed. (E-F) Percent of total cortical parenchyma with interstitial inflammation, interstitial fibrosis/tubular atrophy (IFTA) (E), and the number of arteritis lesions per tissue section (F) was calculated. (G) Glomerular features including mesangial hypercellularity, mesangial deposits, endocapillary hypercellularity, leukocyte infiltration, subendoethlial and intracapillary deposits, crescents, and glomerulosclerosis were graded semiquantitatively on a scale of 0 to 3+ (0, absent; 1+, involving 1%-25% glomeruli; 2+, involving 26%-50% glomeruli; 3+, involving >50% glomeruli per coronal section of kidney containing >150 glomeruli per mouse). (H) Representative histologic PAS-stained kidney sections are shown. Data are cumulative from 2 independent experiments with 10 mice per group. Statistical significance was determined by Mann-Whitney test or repeated measures 2-way ANOVA with Sidak multiple comparisons test: ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001. IgG, immunoglobulin G.
ICPi treatment promotes proinflammatory CD4+ T cells
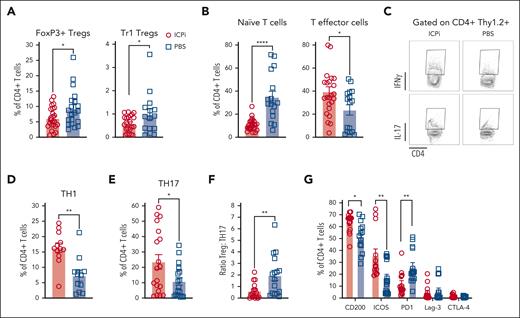
An imbalance between Tregs and proinflammatory T cells may initiate or perpetuate autoimmune diseases.30,33-35 Consistent with prior findings, ∼10% of CD4+ T cells in tolerized HOD+OTII+ mice were FoxP3+ Tregs,9 which were significantly reduced, alongside Tr1 Tregs, in ICPi-treated animals (Figure 4A). Compared with controls, ICPi-treated mice had significantly decreased frequencies of naïve T cells and an expansion of effector T cells (Figure 4B). To assess TH subsets after ICPi treatment, enriched splenic CD4+ T cells were restimulated overnight in vitro. Increased frequencies of proinflammatory IFN-γ+ TH1 and IL-17+ TH17 T cells were observed in ICPi-treated animals, compared with controls (Figure 4C-E). Of note, the Treg:TH17 ratio was significantly decreased in ICPi-treated mice, demonstrating an imbalance between regulatory and proinflammatory T cells (Figure 4F). Because ICPi-treated animals had splenomegaly, absolute CD4+ T-cell numbers for each subset were extrapolated, which were significantly higher than that of controls (supplemental Figure 4A).
ICPi treatment promotes expansion of proinflammatory CD4+T cells. Spleens were collected from ICPi- (red) and PBS-treated (blue) young (aged 2-3 months) HOD+OTII+ mice after 14 days of treatment. Splenocytes were processed into single-cell suspensions and stained with antibodies to delineate T-cell subsets. (A) The frequency of Tregs identified as FoxP3+ Tregs (CD4+CD25+FoxP3+) and Tr1 Tregs (CD4+FoxP3–CD25–CD49b+Lag3+IL10+) of total CD4+ T cells was calculated. To determine Tr1 Tregs, enriched CD4+ T cells were stimulated for 72 hours at 37°C with PMA (50 ng/mL) and ionomycin (500 ng/mL), incubated 1 hour with brefeldin A, and stained to identify the Treg subset. (B) CD4+ T cells were stained with antibodies against CD62L and CD44 to quantify the percentage of CD62L+CD44– naïve and CD62L–CD44+ effector cells. To determine THs, enriched CD4+ T cells were stimulated for 24 hours at 37°C with PMA (50 ng/mL) and ionomycin (500 ng/mL), incubated 1 hour with brefeldin A, and stained intracellularly for IFN-γ and IL-17A. (C-E) Representative flow plots (C) and frequency of TH1 (D) and TH17 cells (E). (F) Ratio of Tregs-to-TH17 cells was calculated for both groups of mice. (G) Frequency of CD4+ T cells expressing CD200, ICOS, PD1, Lag-3, and CTLA-4. Different clones than those contained within the ICPi cocktail were used for PD1, Lag-3, and CTLA-4 staining. Data shown are cumulative of 4 to 5 independent experiments with 5 mice per group. Statistical significance was determined by Mann-Whitney test; ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
ICPi treatment promotes expansion of proinflammatory CD4+T cells. Spleens were collected from ICPi- (red) and PBS-treated (blue) young (aged 2-3 months) HOD+OTII+ mice after 14 days of treatment. Splenocytes were processed into single-cell suspensions and stained with antibodies to delineate T-cell subsets. (A) The frequency of Tregs identified as FoxP3+ Tregs (CD4+CD25+FoxP3+) and Tr1 Tregs (CD4+FoxP3–CD25–CD49b+Lag3+IL10+) of total CD4+ T cells was calculated. To determine Tr1 Tregs, enriched CD4+ T cells were stimulated for 72 hours at 37°C with PMA (50 ng/mL) and ionomycin (500 ng/mL), incubated 1 hour with brefeldin A, and stained to identify the Treg subset. (B) CD4+ T cells were stained with antibodies against CD62L and CD44 to quantify the percentage of CD62L+CD44– naïve and CD62L–CD44+ effector cells. To determine THs, enriched CD4+ T cells were stimulated for 24 hours at 37°C with PMA (50 ng/mL) and ionomycin (500 ng/mL), incubated 1 hour with brefeldin A, and stained intracellularly for IFN-γ and IL-17A. (C-E) Representative flow plots (C) and frequency of TH1 (D) and TH17 cells (E). (F) Ratio of Tregs-to-TH17 cells was calculated for both groups of mice. (G) Frequency of CD4+ T cells expressing CD200, ICOS, PD1, Lag-3, and CTLA-4. Different clones than those contained within the ICPi cocktail were used for PD1, Lag-3, and CTLA-4 staining. Data shown are cumulative of 4 to 5 independent experiments with 5 mice per group. Statistical significance was determined by Mann-Whitney test; ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
In tumor immunotherapy, checkpoint molecule inhibition can induce compensatory upregulation of other immune molecules.36,37 Therefore, enriched splenic CD4+ T cells were stained with antibodies against CD200, ICOS, and with noncompeting clones for PD1, Lag-3, and CTLA-4 used in ICPi. In ICPi-treated mice, CD200 and ICOS expression were significantly increased, whereas PD1 was reduced (Figure 4G; supplemental Figure 4B). Lag-3 and CTLA-4 expression remained unchanged. Together, these data demonstrate dynamic modulation of checkpoint molecule expression in response to the ICPi.
CD39SP predict RBC tolerance failure
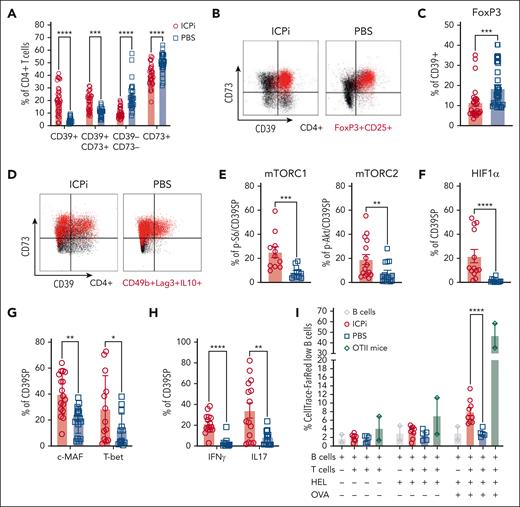
Based on the RNAseq data and the observed upregulation of surface CD73 expression in tolerized, autoreactive CD4+ RTEs, we hypothesized that purinergic signaling molecules are instrumental for RBC autoantigen tolerance. ICPi-treated mice had significant increases in frequencies of CD4+ T cells coexpressing CD39 and CD73, along with reductions in a subset expressing CD73 alone (Figure 5A). However, a population of CD4+ T cells expressing only CD39 was significantly increased in ICPi-treated mice. Because most Tregs coexpress CD73 and CD39,20 we tested whether CD39+ were FoxP3+ or Tr1 Tregs. Although most FoxP3+ Tregs from ICPi-treated mice coexpressed CD39 and CD73, most CD39+ did not express FoxP3 (Figure 5B-C; supplemental Figure 5A-B). Additionally, CD39+ lacked expression of Tr1 Treg markers Lag-3 and CD49b (Figure 5D; supplemental Figure 5C). These data show that CD39+ do not express Treg markers and will be defined phenotypically as CD4+CD39+CD73−FoxP3–CD25– and referred to as CD39SP.
CD39SP exhibits a proinflammatory TH phenotype and stimulates B-cell proliferation. Spleens from ICPi- (red) and PBS-treated (blue) young (aged 2-3 months) HOD+OTII+ mice were collected 14 days after treatment and CD4 enriched. (A) Frequency of T cells expressing purinergic signaling molecules CD39 and CD73. (B) Representative flow plots of expression of CD39 and CD73 on total CD4+Vα2+Vβ5+ OTII T cells (black) and CD4+ FoxP3+CD25+Vα2+Vβ5+ Tregs (red) are shown. (C) Frequency of CD39+ CD4+ T cells expressing FoxP3. (D) Representative flow plots of expression of CD39 and CD73 on CD4+Vα2+Vβ5+ OTII T cells (black) and CD49b+Lag3+IL10+ Tr1 Tregs (red). These data show that CD39+ do not express Treg markers and will be defined phenotypically as CD4+CD39+CD73–FoxP3–CD25– and referred to as CD39SP. (E-H) The frequencies of CD39SP that stained positive for (E) p-S6 and p-Akt, markers of mTOR complexes 1 and 2 activation, (F) HIF1α hypoxia transcription factor, (G) transcription factors c-MAF and T-bet, and (H) proinflammatory cytokines IFN-γ and IL-17 were determined. (I) CD39SP were sorted from ICPi- (red) and PBS-treated (blue) animals and cocultured with B cells at 1:2 ratio, previously labeled with CellTrace-FR and stimulated with HEL or HEL + OVA. CellTrace-FR dilution as measure of B-cell proliferation was assessed 3 days after stimulation. CD4+ T cells enriched from OTII mice (green) were used as a positive control. Data presented in panels A-H are cumulative from 4 to 5 independent experiments with 3 to 4 mice per group. Data shown in panel I are cumulative of 2 independent experiments with 4 to 5 mice per group. Statistical significance was determined by 2-way ANOVA with Sidak posttest and Mann-Whitney test: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. HIF1α, hypoxia inducible factor 1 subunit α; mTORC, mammalian target of rapamycin complex.
CD39SP exhibits a proinflammatory TH phenotype and stimulates B-cell proliferation. Spleens from ICPi- (red) and PBS-treated (blue) young (aged 2-3 months) HOD+OTII+ mice were collected 14 days after treatment and CD4 enriched. (A) Frequency of T cells expressing purinergic signaling molecules CD39 and CD73. (B) Representative flow plots of expression of CD39 and CD73 on total CD4+Vα2+Vβ5+ OTII T cells (black) and CD4+ FoxP3+CD25+Vα2+Vβ5+ Tregs (red) are shown. (C) Frequency of CD39+ CD4+ T cells expressing FoxP3. (D) Representative flow plots of expression of CD39 and CD73 on CD4+Vα2+Vβ5+ OTII T cells (black) and CD49b+Lag3+IL10+ Tr1 Tregs (red). These data show that CD39+ do not express Treg markers and will be defined phenotypically as CD4+CD39+CD73–FoxP3–CD25– and referred to as CD39SP. (E-H) The frequencies of CD39SP that stained positive for (E) p-S6 and p-Akt, markers of mTOR complexes 1 and 2 activation, (F) HIF1α hypoxia transcription factor, (G) transcription factors c-MAF and T-bet, and (H) proinflammatory cytokines IFN-γ and IL-17 were determined. (I) CD39SP were sorted from ICPi- (red) and PBS-treated (blue) animals and cocultured with B cells at 1:2 ratio, previously labeled with CellTrace-FR and stimulated with HEL or HEL + OVA. CellTrace-FR dilution as measure of B-cell proliferation was assessed 3 days after stimulation. CD4+ T cells enriched from OTII mice (green) were used as a positive control. Data presented in panels A-H are cumulative from 4 to 5 independent experiments with 3 to 4 mice per group. Data shown in panel I are cumulative of 2 independent experiments with 4 to 5 mice per group. Statistical significance was determined by 2-way ANOVA with Sidak posttest and Mann-Whitney test: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. HIF1α, hypoxia inducible factor 1 subunit α; mTORC, mammalian target of rapamycin complex.
To test whether CD39SP were metabolically active and determine their effector T-cell subset, mTOR signaling molecules and cytokine production were evaluated. CD39SP from ICPi-treated mice had significantly higher expression of p-S6 and p-Akt, markers of mTOR complexes 1 and 2, respectively, (Figure 5E), and of HIF1α, indicating that CD39SP are metabolically active and may differentiate into TH17 T cells, than controls (Figure 5F).38 Moreover, CD39SP from ICPi-treated mice were more likely to express c-MAF and T-bet transcription factors and produce proinflammatory IFN-γ and IL-17 upon restimulation (Figure 5G-H); no IL4 was detectable (not shown). CD39SP expressed CD62LhighCD44low, a phenotype consistent with T effector cells (supplemental Figure 5D). Together, these data suggest that CD39SP are pathogenic and may initiate and/or perpetuate AIHA.
Up to this point, the data support the concept that CD39SP from ICPi-treated mice exhibit effector/helper, rather than regulatory, T-cell characteristics. To explore whether CD39SP were functional, their ability to provide B-cell help (ie, stimulating proliferation) was evaluated. CD39SP, which are specific for OVA, were sorted from ICPi-treated and naïve HOD+OTII+ mice and cocultured with HEL-specific transgenic B cells in the presence of soluble HEL or HEL plus OVA. CD39SP from ICPi-treated animals, but not naïve, stimulated B-cell proliferation when cocultured with HEL plus OVA, as inferred by decreased CellTrace-Far Red (FR) signal (Figure 5I). No significant B-cell proliferation was observed in B cells alone or with CD39SP from naïve mice. These data demonstrate that ICPi–induced CD39SP activate B cells, suggesting they may perpetuate AIHA disease.
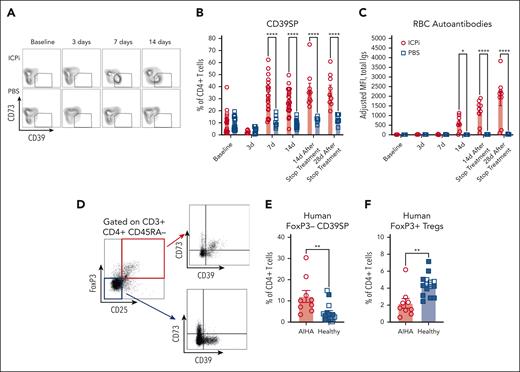
To gain insight into the role of CD39SP in AIHA pathogenesis, the kinetics of CD39SP appearance was monitored in peripheral blood during ICPi treatment. CD39SP appeared 7 days after ICPi treatment (Figure 6A) and were detectable before RBC autoantibodies could be reliably identified (Figure 6B-C). Similar trends were noted in other models, including older HOD+OTII+ mice that had spontaneously produced autoantibodies with aging (supplemental Figure 5E)10 and in ICPi-treated NZB and B6 mice (supplemental Figure 5F-G), suggesting that CD39SP may be an early biomarker that predicts subsequent RBC autoantibody production.
CD39SP precede autoantibody production. Whole blood and sera were collected from young (aged 2-3 months) HOD+OTII+ mice before, during (3 and 7 days), and at the cessation of ICPi or PBS treatment (∼14 days); (A-C) representative flow plots (A) and frequency of CD39SP in peripheral whole blood (B) and RBC autoantibodies (C) in sera are shown at each time point. (D) Representative flow plots of FoxP3 and CD25 staining in CD3+CD4+CD45RA– gated T cells isolated from human patients with AIHA (n = 9) and healthy volunteers (n = 15). Expression of CD39 and CD73 on gated FoxP3+ Tregs (red box) and on gated FoxP3–CD25– T cells (blue box) were shown. (E-F) Frequencies of CD39SP with phenotype CD4+CD39+CD73–FoxP3–CD25– (E) and FoxP3+ Tregs (F) were detected in PBMCs from patients with AIHA (not treated with ICPi) and healthy volunteers. Age-matched healthy volunteers are colored blue. Data presented in panels A-C are cumulative from 4 to 5 independent experiments with 3 to 4 mice per group. Statistical significance was determined by Mann-Whitney test: ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
CD39SP precede autoantibody production. Whole blood and sera were collected from young (aged 2-3 months) HOD+OTII+ mice before, during (3 and 7 days), and at the cessation of ICPi or PBS treatment (∼14 days); (A-C) representative flow plots (A) and frequency of CD39SP in peripheral whole blood (B) and RBC autoantibodies (C) in sera are shown at each time point. (D) Representative flow plots of FoxP3 and CD25 staining in CD3+CD4+CD45RA– gated T cells isolated from human patients with AIHA (n = 9) and healthy volunteers (n = 15). Expression of CD39 and CD73 on gated FoxP3+ Tregs (red box) and on gated FoxP3–CD25– T cells (blue box) were shown. (E-F) Frequencies of CD39SP with phenotype CD4+CD39+CD73–FoxP3–CD25– (E) and FoxP3+ Tregs (F) were detected in PBMCs from patients with AIHA (not treated with ICPi) and healthy volunteers. Age-matched healthy volunteers are colored blue. Data presented in panels A-C are cumulative from 4 to 5 independent experiments with 3 to 4 mice per group. Statistical significance was determined by Mann-Whitney test: ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Because the role of purinergic signaling in AIHA has yet to be described in humans, we tested whether findings from our preclinical model could be extended to patients. Remarkably, CD39SP were detectable at higher frequencies in PBMCs from patients with AIHA than healthy controls (Figure 6D-E), whereas FoxP3+ Tregs were lower in patients (Figure 6F), further supporting the importance of the purinergic signaling pathway in AIHA and overall translatability of our findings.
Apyrase treatment mitigates ICPi-induced AIHA
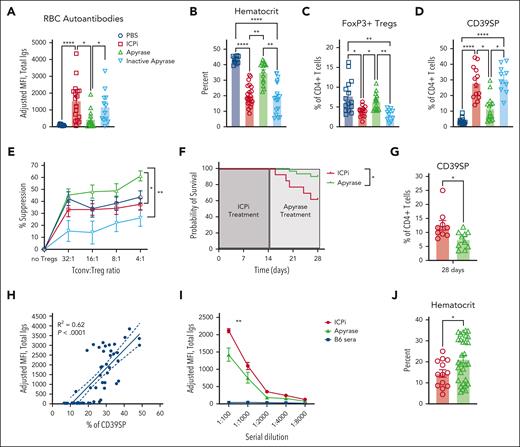
Extracellular ATP (eATP) is a proinflammatory signal and, together with decreased levels of immunosuppressive adenosine, can potentially induce autoimmunity.39 To test whether eATP plays a role in RBC autoantibody production, before exposure to ICPi, mice were prophylactically injected with apyrase, an enzyme that degrades ATP. Mice that received apyrase before every infusion of ICPi had significant reductions in RBC autoantibody levels and increased hematocrit, compared with controls (Figure 7A-B). This effect depended upon infusion of functional enzyme, because mice given heat-inactivated apyrase14 produced RBC autoantibody levels comparable with mice treated with ICPi alone. Apyrase also prevented FoxP3+ Treg reductions (Figure 7C) and blunted CD39SP expansion (Figure 7D). Lastly, using an in vitro suppression assay, enriched CD4+CD25+ T cells from apyrase-treated mice exhibited a significantly increased capacity to suppress conventional T cells, compared with T cells from animals treated with ICPi alone or with inactivated apyrase (Figure 7E), which may be explained by upregulation of checkpoint molecule expression (supplemental Figure 5H).
Prophylactic and therapeutic administration of apyrase ameliorates ICPi-induced AIHA. Whole blood was collected from young (aged 2-3 months) HOD+OTII+ mice treated with apyrase or with an inactivated apyrase before every ICPi injection. (A-B) Sera were analyzed for RBC autoantibodies by flow crossmatch for HOD-specific autoantibodies (A) and hematocrit percentage (B) was calculated. (C-D) Frequencies of FoxP3+ Tregs (C) and CD39SP (D) were calculated. (E) Suppressive activity of CD4+CD25+ T cells collected from apyrase-pretreated (green), inactive apyrase-pretreated (light blue), ICPi-treated (red), and PBS-treated (blue) HOD+OTII+ mice. Suppression was assessed by adding CD4+CD25− T cells (Tconv) in culture with serial dilutions of CD4+CD25+ enriched T cells. Cells were stimulated with anti-CD3 10 μg/mL and anti-CD28 20 μg/mL for 72 hours at 37°C. Suppression was expressed as percentage of decreased number of Tconv. (F-G) Kaplan-Meier survival curve (F) and CD39SP level (G) in whole blood of mice treated with ICPi for 14 days followed by injection of PBS (red line) or 5 units of apyrase daily for 14 days (green line). (H) Linear regression analysis showing a significant positive correlation between RBC autoantibody levels and frequency of CD39SP (R2 = 0.62; P < .0001). (I) Young (aged 2-3 months) HOD+OTII+ mice were treated with ICPi for 14 days alone (red; n = 13) or with apyrase treatment beginning at 7 days (ie, when CD39SP are detectable; green; n = 30). Sera from B6 animals were collected as controls (blue). Sera were serially diluted and analyzed for RBC autoantibodies then analyzed in a flow crossmatch. (J) Hematocrit percentage for mice treated with ICPi alone or with apyrase was calculated. Data presented in panels A-D are combined from 3 different experiments with 4 mice per group. Data presented in panels E-F are combined from 2 different experiments. Statistical significance was determined by 2-way ANOVA with Sidak posttest, Mann-Whitney test, and by log-rank test: ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Prophylactic and therapeutic administration of apyrase ameliorates ICPi-induced AIHA. Whole blood was collected from young (aged 2-3 months) HOD+OTII+ mice treated with apyrase or with an inactivated apyrase before every ICPi injection. (A-B) Sera were analyzed for RBC autoantibodies by flow crossmatch for HOD-specific autoantibodies (A) and hematocrit percentage (B) was calculated. (C-D) Frequencies of FoxP3+ Tregs (C) and CD39SP (D) were calculated. (E) Suppressive activity of CD4+CD25+ T cells collected from apyrase-pretreated (green), inactive apyrase-pretreated (light blue), ICPi-treated (red), and PBS-treated (blue) HOD+OTII+ mice. Suppression was assessed by adding CD4+CD25− T cells (Tconv) in culture with serial dilutions of CD4+CD25+ enriched T cells. Cells were stimulated with anti-CD3 10 μg/mL and anti-CD28 20 μg/mL for 72 hours at 37°C. Suppression was expressed as percentage of decreased number of Tconv. (F-G) Kaplan-Meier survival curve (F) and CD39SP level (G) in whole blood of mice treated with ICPi for 14 days followed by injection of PBS (red line) or 5 units of apyrase daily for 14 days (green line). (H) Linear regression analysis showing a significant positive correlation between RBC autoantibody levels and frequency of CD39SP (R2 = 0.62; P < .0001). (I) Young (aged 2-3 months) HOD+OTII+ mice were treated with ICPi for 14 days alone (red; n = 13) or with apyrase treatment beginning at 7 days (ie, when CD39SP are detectable; green; n = 30). Sera from B6 animals were collected as controls (blue). Sera were serially diluted and analyzed for RBC autoantibodies then analyzed in a flow crossmatch. (J) Hematocrit percentage for mice treated with ICPi alone or with apyrase was calculated. Data presented in panels A-D are combined from 3 different experiments with 4 mice per group. Data presented in panels E-F are combined from 2 different experiments. Statistical significance was determined by 2-way ANOVA with Sidak posttest, Mann-Whitney test, and by log-rank test: ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Apyrase was also evaluated therapeutically by treating mice with ongoing RBC autoantibody production (ie, 14 days after ICPi). Although anemia and autoantibody levels were not ameliorated (not shown), apyrase treatment enhanced survival rates, compared with control mice given PBS (Figure 7F). Similar to prophylactic administration, therapeutic apyrase led to significant reductions of CD39SP in ICPi-treated mice (Figure 7G); CD39SP persisted at least 2 weeks ICPi and correlated with autoantibody levels (Figure 7G-H). Because increased CD39SP predicted tolerance failure and appeared before detectable RBC autoantibodies, we tested the effect of apyrase treatment at the time of CD39SP detection (but before autoantibody formation). Apyrase-treated mice had significant reductions in RBC autoantibody levels and increased hematocrit, compared with controls (Figure 7I-J). These findings demonstrate that reducing eATP levels with apyrase decreased AIHA development and augmented Treg function, suggesting a promising role for apyrase in attenuating ICPi-induced RBC autoantibodies and improving overall survival.
Discussion
AIHA, both spontaneous and secondary to ICPi therapy, causes significant morbidity and mortality in humans. We recently reported that CD4+ T-cell tolerance to RBC antigens occurs in the periphery, within 3 weeks after thymic egress,9 highlighting the crucial role of peripheral tolerance in preventing RBC autoimmunity. These findings were generated in a novel AIHA murine model that mirrors human idiopathic AIHA.10 Herein, we adapted this system to model ICPi-AIHA. This study advances understanding at 3 levels. First, we extended our previous correlative observations of specific immunoregulatory pathways to a direct causal role in preventing AIHA. Second, we established that ICPi-AIHA is preceded by, and associated with, increased CD39SP. Finally, we report that depriving CD39SP of its ATP substrate, with apyrase, mitigates ICPi-AIHA. Although these mechanistic advances were made in a mouse model, we confirmed an association of increased CD39SP and AIHA in humans. The extent to which the increases in CD39SP predicts AIHA in humans will have to be tested empirically in subsequent human studies.
Active suppression of autoimmunity has not been considered important in RBC tolerance, because CD25-mediated Treg depletion does not induce RBC autoantibodies in a mouse model.40 However, the current observation of 2 distinct Treg populations raises the possibility that FoxP3+ Tregs and Tr1 cells are redundant mechanisms of tolerance, with neither being necessary to prevent AIHA and either being sufficient. The importance of FoxP3+ Tregs in regulating human AIHA is unclear. Approximately 50% of humans with congenital FOXP3 loss-of-function mutations (ie, immunodysregulation, polyendocrinopathy, enteropathy, X linked [IPEX]) present with AIHA.41,42 This suggests that FoxP3+ Tregs play an essential role in maintaining tolerance to RBC autoantigens in some humans but are not essential in all humans. Our data support a hypothesis that humans missing FoxP3+ Tregs without developing AIHA have higher levels of compensatory Tr1 cells. Indeed, patients with IPEX still generate Tr1,43,44 and Tr1 have been proposed to have an important role in preventing AIHA. To our knowledge, there are no available data to simultaneously assess FoxP3+ and Tr1 Treg levels in humans with AIHA, either in IPEX or due to other etiologies.
Proinflammatory TH1 and TH17 expansion was observed coincident with decreased FoxP3+ and Tr1 Tregs. TH17 cells play a crucial role in immune-mediated diseases and have links to AIHA severity in human patients and murine models.31,35,45,46 In patients with AIHA, FoxP3 downregulation correlates with decreased FoxP3+ Treg frequencies and expanded TH17 cells.30 Collectively, our data suggest that reducing 2 Treg subsets while increasing proinflammatory TH1 and TH17 cells promotes AIHA. The origin of the TH1 and TH17 cells are unclear but may include de novo generation and/or differentiation of Tregs into TH1 and TH17.
Tregs express high levels of CD39 and CD73. CD39 is an ectonuclease enzyme that, in cooperation with CD73, hydrolyzes eATP into adenosine.20 ATP can be proinflammatory, and chronically high eATP levels promote inflammation, inhibit FoxP3 expression, and can convert Tregs into TH17.46 In contrast, adenosine can be immunosuppressive, promote Treg generation, and prevent T-cell activation and proliferation.25,39 Thus, the coexpression of CD39 and CD73 facilitates the conversion of proinflammatory eATP into immunosuppressive adenosine.20 Unexpectedly, a CD4+ T-cell subset expressing CD39 without CD73 (ie, CD39SP) emerged in ICPi-AIHA. Although we cannot rule out that CD39SP arose from unstable Tregs,47,48 CD39SP lacked FoxP3 and Tr1 markers and expressed transcription factors and secreted cytokines most consistent with inflammatory TH1 and/or TH17 T cells.
CD39SP appeared before detectable RBC autoantibodies and stimulated B-cell proliferation, suggesting they predict autoimmunity onset and may contribute to AIHA pathology. In addition to our ICPi-AIHA model, CD39SP were observed in our age-onset AIHA model and in ICPi-treated NZB and healthy B6 mice, suggesting that CD39SP is a generalizable predictor of AIHA, regardless of etiology. Lastly, CD39SP were detectable in patients with secondary AIHA, without ICPi treatment, supporting the general association of CD39SP with RBC autoantibodies. Of note, patient samples were procured after AIHA diagnosis and medication administration, and additional studies are needed to evaluate the extent to which CD39SP predicts AIHA in humans.
The predictive power of CD39SP cells is made far more relevant considering the observation that apyrase was effective if administered at the time of CD39SP appearance before autoantibody production. Importantly, although CD39SP frequencies persisted in untreated mice, they significantly decreased in apyrase-treated animals, demonstrating CD39SP are responsive to therapy and may provide a surrogate for therapeutic efficacy. We also detected a significant correlation between CD39SP and AIHA severity. Together, these data suggest that CD39SP analysis in PBMCs in patients undergoing ICPi therapy may enable early AIHA detection, facilitating timely intervention. Furthemore, because current AIHA therapies have variable success,27-29 CD39SP monitoring may serve as an early predictor of potential treatment efficacy.
This study has several limitations, particularly regarding the AIHA mouse model used, which has supraphysiological levels of RBC autoreactive CD4+ T cells, enabling the study of antigen-specific responses. However, neither NZB nor B6 mice have supraphysiological levels of RBC autoreactive T cells, yet the same ICPi-AIHA was observed. Thus, it is unlikely that increased RBC autoreactive T-cell frequency confounded the overall biology being studied. Second, our use of an ICPi cocktail targeting multiple checkpoints was designed to test causal roles of immunoregulatory molecules detected by unbiased screening. This differs from single-drug treatments, which have not been tested in this model; of note, tolerance fails within 2 weeks of treatment in these mice, compared with a 55-day average in patients receiving ICPi.49 Considering the complexity of multiple checkpoints and Treg populations, especially in the context of age- and sex-related factors influencing AIHA development, it is uncertain whether single-agent therapy would elicit as robust a response as the current combination, particularly in young animals. Finally, similar to any animal model, there is the risk that the findings will not translate into human biology. However, CD39SP are detectable in PBMCs from patients with AIHA, suggesting at least an analogous cellular biology.
In summary, our studies introduce a novel mouse model of ICPi-AIHA, a well-documented irAE in patients with cancer undergoing checkpoint inhibitor therapy. This model offers valuable mechanistic insights into tolerance breakdown, revealing a reduction in 2 distinct Treg subsets and an expansion of proinflammatory T cells. Our investigations unveil a previously unrecognized role for the purinergic signaling pathway in shaping RBC autoantigen tolerance, highlighting CD39SP as a predictive biomarker for tolerance failure and identifying apyrase as an effective therapeutic intervention. Furthermore, our findings extend beyond this model, because CD39SP are detectable in multiple mouse models developing AIHA and patients with AIHA, suggesting broad applicability to both idiopathic AIHA and AIHA secondary to other conditions.
Acknowledgments
The authors thank James C. Zimring (University of Virginia), Steven L. Spitalnik (Columbia University), and Eldad A. Hod (Columbia University) for intellectually stimulating discussions and critical review of the manuscript. The authors thank the staff of the Columbia Stem Cell Initiative Flow Cytometry Core Facility, under the leadership of Michael Kissner, at Columba University Irving Medical Center for their contributions to the work presented in this manuscript.
The Sony MA9000 (Venus) was purchased with funds from the Milky Way Research Foundation. This study was supported by the National Institutes of Health, including the National Heart, Lung, and Blood Institute and the Office of Autoimmune Disease Research in the Office of Research on Women’s Health (grant R01HL133325 [K.E.H.]).
Authorship
Contribution: F.D.Z., A.Q., S.J., E.K., M.T., and A.M. performed experiments and analyzed the data; V.D.D. performed the histologic analysis and interpretation of kidney pathology; D.F., E.F.S., and S.P. collected samples from human patients and volunteers; K.E.H. and F.D.Z. conceived and supervised the project and wrote the paper; and all authors edited the manuscript and approved of the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Krystalyn E. Hudson, Department of Pathology and Cell Biology, Laboratory of Transfusion Biology, Columbia University Irving Medical Center, 630 W 168th St, Room P&S 15-401, New York, NY 10032; email: keh2197@cumc.columbia.edu.
References
Author notes
All data necessary to understand and evaluate the conclusions of this manuscript are provided in the article, its source data, and supplemental information. All RNA sequencing raw data and additional information on library construction, data processing, and data analysis have been deposited in Dryad and can be accessed at https://doi.org/10.5061/dryad.5qfttdzdf.
Any other data can be obtained upon reasonable request from the corresponding author, Krystalyn E. Hudson (keh2197@cumc.columbia.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal