Key Points
sGPVI, a platelet-derived blood biomarker, is predictive of AAAs, with high specificity in distinguishing patients with rapidly growing AAA.
Blockade of GPVI in mice with established aneurysms reduces AAA progression and mortality, indicating therapeutic potential.
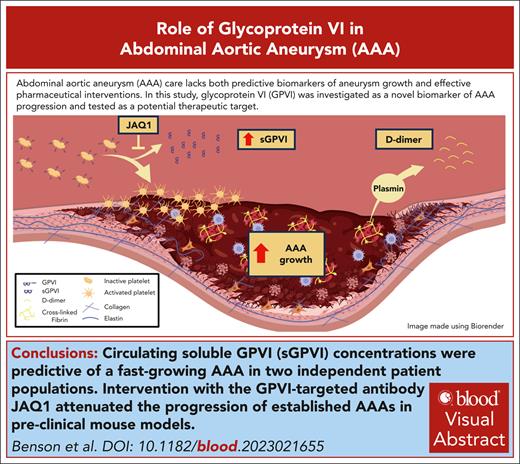
Visual Abstract
A common feature in patients with abdominal aortic aneurysms (AAAs) is the formation of a nonocclusive intraluminal thrombus (ILT) in regions of aortic dilation. Platelets are known to maintain hemostasis and propagate thrombosis through several redundant activation mechanisms, yet the role of platelet activation in the pathogenesis of AAA-associated ILT is still poorly understood. Thus, we sought to investigate how platelet activation affects the pathogenesis of AAA. Using RNA sequencing, we identified that the platelet-associated transcripts are significantly enriched in the ILT compared with the adjacent aneurysm wall and healthy control aortas. We found that the platelet-specific receptor glycoprotein VI (GPVI) is among the top enriched genes in AAA ILT and is increased on the platelet surface of patients with AAAs. Examination of a specific indicator of platelet activity, soluble GPVI (sGPVI), in 2 independent cohorts of patients with AAAs is highly predictive of an AAA diagnosis and associates more strongly with aneurysm growth rate than D-dimer in humans. Finally, intervention with the anti-GPVI antibody (JAQ1) in mice with established aneurysms blunted the progression of AAA in 2 independent mouse models. In conclusion, we show that the levels of sGPVI in humans can predict a diagnosis of AAA and AAA growth rate, which may be critical in the identification of high-risk patients. We also identify GPVI as a novel platelet-specific AAA therapeutic target, with minimal risk of adverse bleeding complications, for which none currently exists.
Introduction
Abdominal aortic aneurysm (AAA) is defined as a localized degeneration of the aortic wall resulting in >50% permanent dilation, hallmarked by progressive inflammatory infiltrate and medial wall degradation.1,2 With >1 million American adults affected by the disease, the prevalence is ∼2% to 3% in males and 0.5% to 1% of females aged >65 years, resulting in ∼9928 deaths per year in the United States.3 Currently, only surgical repair for large (≥55 mm) or rapidly expanding aneurysms is available to prevent aortic rupture, a frequently fatal event.4 Understanding the multifactorial complexities of AAA and identifying high-risk patients with predictive biomarkers are necessary to improve treatment strategies for patients with aneurysm.
A prominent feature of AAA pathogenesis is the formation of a nonocclusive intraluminal thrombus (ILT), driven by increased platelet activity and impaired hemostasis.5,6 Although ILT formation may stabilize the aorta during AAA initiation,7 there is increasing evidence that the ILT contributes to localized inflammation, medial degeneration, and progressive immune infiltration.2 Patients with large and small AAAs have significant increases in circulating hemostatic markers including platelet factor 4, thrombin-antithrombin complex, and D-dimer.8 Although there are no recognized gold-standard biomarkers for AAAs or progression to rupture, the most established is D-dimer, which is produced as a result of fibrinolysis and indicates active thrombus formation and degradation.9,10 A longitudinal study of 299 patients with AAA demonstrated D-dimer was significantly associated with AAA growth rate,11 whereas another found D-dimer correlated with subaneurysmal dilation.12 Conversely, our previous analysis of patients with AAA indicated that although D-dimer was associated with AAA incidence, no significant difference was observed between fast- (defined as ≥2 mm/y) or slow-growing (<2 mm/y) aneurysms.8 Although D-dimer could potentially be used as a biomarker for AAA status, its usefulness for predicting AAA progression and rupture remains uncertain.
Platelets are critical to the formation and propagation of ILT and are largely present at the luminal surface among trapped erythrocytes, neutrophils, and a dense fibrin network.7,13 The platelet-specific receptor glycoprotein VI (GPVI) binds collagen, resulting in platelet activation and adhesion.14-17 GPVI interaction with the D-domain of fibrinogen/fibrin augments platelet adhesion and aggregation and facilitates platelet recruitment to an existing clot.18-21 Once engaged, GPVI triggers rapid platelet activation via the engagement of immunoreceptor tyrosine-based activation motifs within the noncovalently linked Fc receptor γ-chain dimer to convey ligand-mediated signaling, leading to platelet degranulation.22,23 Activation also triggers autologous proteolysis of GPVI mediated by endogenous ADAM10 in human platelets, liberating a 55-kDa soluble ectodomain fragment known as soluble GPVI (sGPVI).24,25 Constituting the only bona fide platelet-specific biomarker of platelet activation,25 sGPVI is elevated in prothrombotic conditions including acute ischemic stroke, sepsis, disseminated intravascular coagulation, and deep vein thrombosis.26 Although traditional antiplatelet therapies may increase bleeding risk in patients with AAA,7,27-29 targeting of GPVI in murine models reduces arterial thrombosis without significantly changing bleeding times.30 Moreover, patients with a rare homozygous deletion of GPVI present with only mild bleeding diathesis,31,32 and genetic deletion of GPVI is not associated with spontaneous bleeding or impaired hemostasis in mice, suggesting GPVI may be a therapeutic target in disorders regulated by activated platelets without affecting hemostasis.21 Early phase clinical trials using antibody-mediated inhibition of GPVI have not reported a significant bleeding phenotype.33-35 Thus, GPVI is a promising target to reduce platelet-mediated thrombosis while avoiding hemorrhagic consequences.
Methods
Comprehensive methods can be found in supplemental Materials, available on the Blood website.
Mouse study approvals
Mouse studies (protocol numbers 15-01-29-01 or 20-11-05-02) were performed with the approval of the University of Cincinnati Institutional Animal Care and Use Committee.
Mice
Male low-density lipoprotein receptor–deficient (Ldlr−/−; B6.129S7-Ldlrtm1Her/J; stock 2207) and C57BL/6J mice (stock 000664) were obtained from The Jackson Laboratory (Bar Harbor, ME).
AngII model of AAA
Male Ldlr−/− mice (n = 190) were infused with sterile saline or angiotensin II (AngII) (1000 ng/kg per minute) via a subcutaneous osmotic minipump (Alzet Corp) for 28 days, as previously described.36
Topical elastase model of AAA
Measurements of abdominal aortic diameters
Enzyme-linked immunosorbent assays and cytokine array
Human sGPVI was measured using a solid-phase rabbit anti-GPVI polyclonal antibody and fluid-phase murine anti-GPVI monoclonal antibody (1A12) as previously described.41
JAQ1 antibody intervention
At 14 days after model initiation, mice with established AAAs were blindly randomized and received a 1-time 250-μL intraperitoneal injection of JAQ1 (50 μg) GPVI neutralizing antibody (EMFRET Analytics GmbH & Co. KG, Eibelstadt, Germany) or rat immunoglobulin G2a (IgG2a; 50 μg) placebo, as described previously.30
Histological processing of abdominal aortas
Histological processing and staining of aortic sections for CD68 Alexa Fluor (Bio-Rad, MCA1957-A647) and Picrosirius red (PolyScientific, Inc) was completed as previously described.42
Human aneurysm samples
Human AAA tissue was obtained from 4 patients (4 males) aged 65 ± 7.7 years (mean ± standard deviation [SD]; range, 45-71) undergoing open aneurysm repair (between 2015 and 2018) at the University of Rochester Medical Center in Rochester, NY, under an approved University of Rochester Medical Center (URMC) Cardiovascular Tissue Bank Institutional Review Board (IRB; Research Subjects Review Board [RSRB] 00036669). Control tissue was procured from the infrarenal aortas of 5 males aged 58 ± 6.6 years (mean ± SD; range, 42-65) with cause of death deemed nonaortic.
RNA processing and sequencing
Human platelet collection
Volunteer control patients (n = 4) with no known medical history, aneurysmal disease, or on antiplatelet agents were recruited in studies approved by the Cleveland Clinic RSRB. Washed platelets from patients with AAA (n = 5) were collected by venipuncture and isolated from citrate plasma from preoperative patients under serial imaging surveillance. Platelet collection, immunoblotting, agonist stimulation, and flow cytometry were performed as described previously.45,46
Research statistics and data representation
Unless indicated otherwise, all bar, scatter, and line graphs were created with Sigma Plot v.14.5 (Statistical Package for the Social Sciences [SPSS], Chicago, IL). Data are represented as individual data points and means ± standard error of the mean. Data normality was assessed using a Shapiro Wilk test. Two group comparisons with assumed normality were performed via Student t test. Nonnormal data were analyzed with a Mann-Whitney rank sum test. Multiple group significance was assessed by 1-way analysis of variance (ANOVA) on ranks with a Dunn post hoc, 1-way ANOVA with Holm-Sidak post hoc, or 2-way ANOVA with Holm-Sidak post hoc, when appropriate. Statistical significance among temporal groups was assessed by either a 1-way repeated measures ANOVA (parametric) or repeated measures ANOVA on ranks (nonparametric), when appropriate. P values <.05 were considered statistically significant.
European study population
American study population
sGPVI, D-dimer, and AAAs were also examined in healthy control or patients with AAA from a US case-control study. Details for this cohort have been previously published and are available in the online supplemental.42
All mouse studies (protocol number 15-01-29-01 and renewal protocol number 20-11-05-02) were performed with approval of the University of Cincinnati Institutional Animal Care and Use Committee. Human AAA tissue was obtained from 4 patients (4 males) aged 65 ± 7.7 years (mean ± SD; range, 45-71) undergoing open aneurysm repair (between 2015 and 2018) at the University of Rochester Medical Center in Rochester, NY, under an approved URMC Cardiovascular Tissue Bank IRB (RSRB 00036669). Control tissue (under the same IRB) was procured from the infrarenal aortas of 5 males aged 58 ± 6.6 years (mean ± SD; range, 42-65) with cause of death deemed nonaortic. Volunteer control patients (n = 4) with no known medical history, aneurysmal disease, or on antiplatelet agents and patients with AAA (n = 5) were recruited in studies approved by the Cleveland Clinic RSRB.
All studies regarding human participants comply with the Declaration of Helsinki. Human AAA tissue was obtained from the University of Rochester Medical Center in Rochester, NY, under an approved University of Rochester Cardiovascular Tissue Bank Internal Review Board Application (RSRB 00036669). All European research was approved by the research ethics review board of the Uppsala/Örebro region (authorization/protocol number Dnr 2007/052). All North American research, including analysis of platelets from healthy individuals, and from patients with AAA, as well as blood biomarkers, were approved by the Cleveland Clinic IRB (protocol number 15834-2015). All participants (European and North American) gave written informed consent before their participation and sample collection. All patients and controls were of similar ethic origin (European and Caucasian) and socioeconomic backgrounds.
Results
Platelet-associated genes are enriched in the ILT of human AAAs
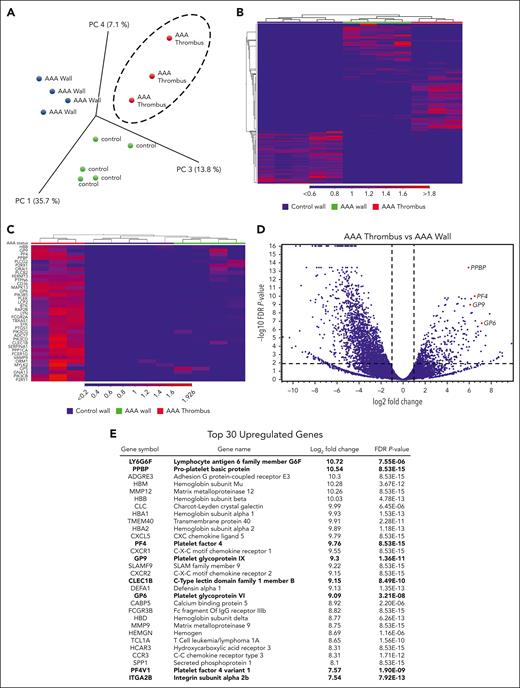
To identify platelet transcripts that may play a role in the pathogenesis of AAA, RNA sequencing was performed on age-matched control aortic tissue (n = 5), AAA aortic tissue (n = 4), and AAA ILT (n = 3). All 3 groups demonstrate spatial separation via principle component analysis (Figure 1A) and heat map clustering (Figure 1B), with many unique sets of differentially expressed genes in each tissue comparison (supplemental Figure 1A). Platelet-associated transcripts clustered within the AAA thrombus vs the AAA aortic tissue (Figure 1C). Differential expression analysis of the AAA thrombus vs the AAA wall (Figure 1D) and control aortic wall (supplemental Figure 1B) demonstrates several platelet transcripts in the upper quartile of significantly enriched genes (noted with red dots), with the volcano plot revealing several significantly upregulated platelet-derived transcripts (Figure 1D, red dots). Platelet transcripts comprise 27% of the top 30 significantly enriched genes in the AAA thrombus vs AAA wall (Figure 1E), including GPVI. Similarly, upon reanalysis of human platelet RNA extracted from healthy individuals (n = 7) and patients with AAA (n = 6) with messenger RNA sequencing, several of these transcripts are also upregulated, including GPVI (supplemental Figure 1C).
Platelet-associated gene expression is enriched in human AAA thrombi. RNA recovered from age-matched control aortic wall (n = 5), AAA aortic wall (n = 4), and AAA thrombi (n = 3) underwent RNA sequencing (RNA-seq). (A) Representation of principal component analysis of the RNA-seq results from control wall, AAA wall, and AAA thrombus samples. (B) Heat map of differences in gene expression in control wall, AAA wall, and AAA thrombus samples. (C) Heat map of platelet-associated transcripts in AAA thrombus vs AAA and control aortic tissue. (D) Volcano plot showing total fold change and P value of significant AAA thrombus genes vs AAA wall, with highlighted platelet-associated genes (red dots). (E) A list of the top 30 significantly enriched genes in AAA thrombus vs AAA wall; platelet-associated genes are bolded. PC, principal component (analysis); PF4, platelet factor 4.
Platelet-associated gene expression is enriched in human AAA thrombi. RNA recovered from age-matched control aortic wall (n = 5), AAA aortic wall (n = 4), and AAA thrombi (n = 3) underwent RNA sequencing (RNA-seq). (A) Representation of principal component analysis of the RNA-seq results from control wall, AAA wall, and AAA thrombus samples. (B) Heat map of differences in gene expression in control wall, AAA wall, and AAA thrombus samples. (C) Heat map of platelet-associated transcripts in AAA thrombus vs AAA and control aortic tissue. (D) Volcano plot showing total fold change and P value of significant AAA thrombus genes vs AAA wall, with highlighted platelet-associated genes (red dots). (E) A list of the top 30 significantly enriched genes in AAA thrombus vs AAA wall; platelet-associated genes are bolded. PC, principal component (analysis); PF4, platelet factor 4.
The platelet-specific collagen receptor GPVI is more active in patients with AAA
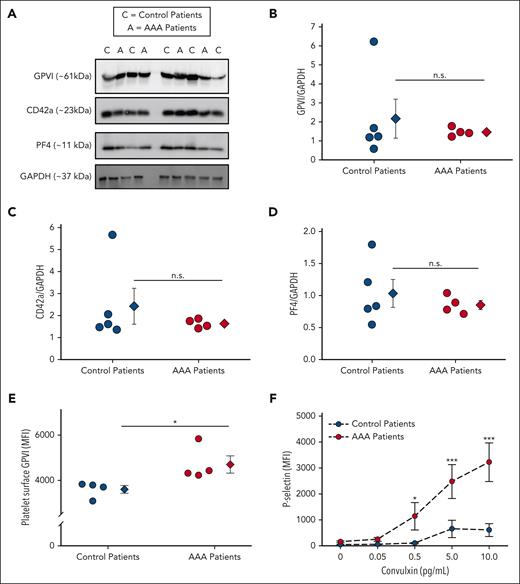
Analysis of a small cohort of human patients with AAA vs age-matched controls did not demonstrate a significant increase in GPVI, platelet factor 4, or CD42a protein via western blotting (Figure 2A-D). Interestingly, flow cytometry revealed a significant increase in surface GPVI, and convulxin-treated platelets, a GPVI specific agonist, from patients with AAA had augmented P-selectin expression compared with controls (Figures 2E-F).47 These data demonstrate patients with AAA are more reactive to GPVI-mediated platelet activation.
The thrombotic platelet receptor GPVI is more active in human patients with AAA. Platelets from control patients (n = 5) and patients with AAA (n = 4) were analyzed for the platelet receptor GPVI. Platelet GPVI, platelet factor 4 (PF4), and CD42a protein expression was measured by western blot (A) and quantified by densitometry (B-D). GPVI on the platelet surface is increased in patients with AAA vs control (E) as demonstrated by platelet surface flow cytometry (∗P = .039 by Student t test). (F) Platelets isolated from patients with AAA demonstrated significantly more surface P-selectin in response to the GPVI specific agonist convulxin than control as measured by flow cytometry (∗P = .046; ∗∗∗P < .001, patients with AAA vs control patients by 2-way ANOVA). Data are represented as individual points or mean ± standard error of the mean (SEM). MFI, mean fluorescence intensity; n.s, not significant.
The thrombotic platelet receptor GPVI is more active in human patients with AAA. Platelets from control patients (n = 5) and patients with AAA (n = 4) were analyzed for the platelet receptor GPVI. Platelet GPVI, platelet factor 4 (PF4), and CD42a protein expression was measured by western blot (A) and quantified by densitometry (B-D). GPVI on the platelet surface is increased in patients with AAA vs control (E) as demonstrated by platelet surface flow cytometry (∗P = .039 by Student t test). (F) Platelets isolated from patients with AAA demonstrated significantly more surface P-selectin in response to the GPVI specific agonist convulxin than control as measured by flow cytometry (∗P = .046; ∗∗∗P < .001, patients with AAA vs control patients by 2-way ANOVA). Data are represented as individual points or mean ± standard error of the mean (SEM). MFI, mean fluorescence intensity; n.s, not significant.
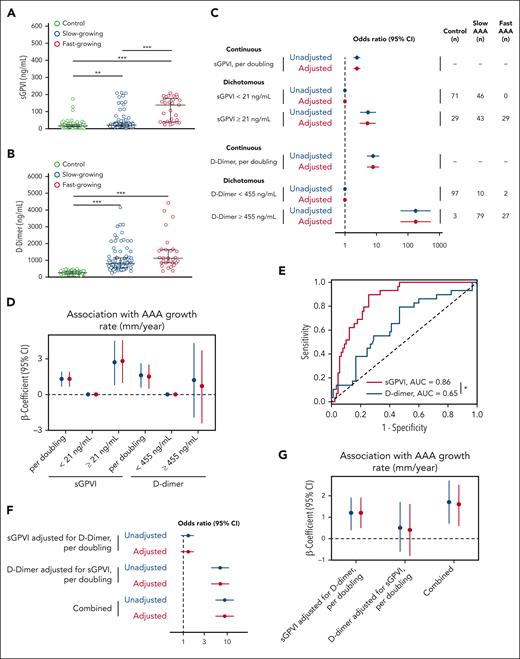
The platelet marker sGPVI and the fibrinolytic marker D-dimer are elevated in patients with AAA and correlate with AAA growth rate
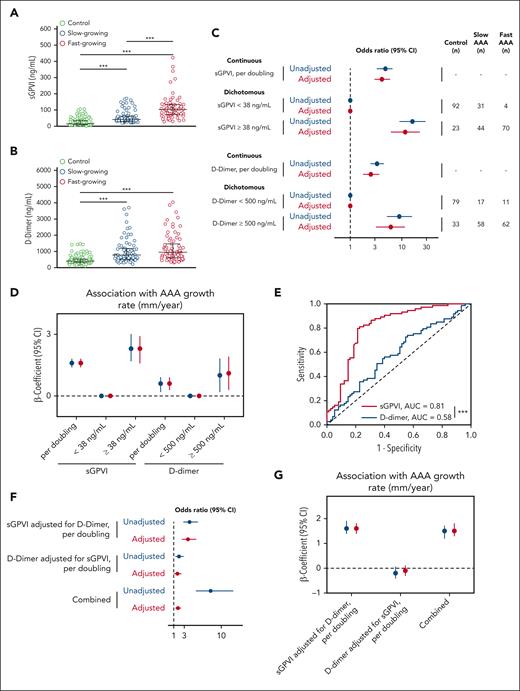
We examined sGPVI concentration in a European cohort of control patients compared with slow- or fast-growing AAAs (Table 1). The predictive value of sGPVI for AAA incidence and growth rate was compared with our published analyses of D-dimer through individual and combined modeling.8 Patients with AAA had significantly higher circulating levels of both sGPVI and D-dimer than control patients, however, only sGPVI was significantly elevated in patients with fast-growing vs those with slow-growing AAA (Figure 3A-B). Ordinal logistic regression demonstrates the odds of being in a higher outcome group (slow or fast AAA) vs a lower outcome group (control), after adjustment, per doubling of sGPVI was 4.1 (95% confidence interval [CI], 3.0-5.8). In dichotomous analysis, for participants with sGPVI levels above the cohort median of 38 ng/mL, the odds of being in a higher outcome group was 11.6 (95% CI, 6.2-21.9) after adjustment (Figure 3C, top). Per doubling of D-dimer, after adjustment, the odds of being in the higher outcome group was 2.5 (95% CI, 1.8-3.6), and it was 6.1 (95% CI, 3.2-11.3) in participants with D-dimer levels above the cohort median of 500 ng/mL after adjustment (Figure 3C, bottom).
Participant characteristics by case definition (n = 264)
| Characteristics . | Control (n = 115) . | Slow-growing AAA (n = 75) . | Fast-growing AAA (n = 74) . |
|---|---|---|---|
| Age, y | 67.4 (2.6) | 72.7 (6.6) | 72.6 (6.8) |
| Height, cm | 177.1 (10.3) | 181.8 (6.2) | 180.7 (6.2) |
| Weight, kg | 84.1 (13.6) | 90.2 (15.7) | 89.3 (16.2) |
| CHD | 10 (8.7) | 27 (36.0) | 25 (33.8) |
| Hypertension | 56 (48.7) | 51 (68.0) | 44 (59.5) |
| CVD | 5 (4.4) | 18 (24.0) | 7 (9.5) |
| Claudication | 1 (0.9) | 8 (10.7) | 1 (1.4) |
| COPD | 5 (4.4) | 12 (16.0) | 11 (14.9) |
| Renal insufficiency | 2 (1.7) | 4 (5.3) | 4 (5.4) |
| Diabetes | 17 (14.8) | 17 (22.7) | 4 (5.4) |
| Aspirin use | 17 (14.8) | 37 (49.3) | 33 (44.6) |
| Statin use | 30 (26.1) | 35 (46.7) | 27 (36.5) |
| Current smoker | 6 (5.2) | 12 (16.0) | 25 (33.8) |
| Smoking years | 23.4 (13.7) | 34.2 (13.0) | 39.4 (14.3) |
| Characteristics . | Control (n = 115) . | Slow-growing AAA (n = 75) . | Fast-growing AAA (n = 74) . |
|---|---|---|---|
| Age, y | 67.4 (2.6) | 72.7 (6.6) | 72.6 (6.8) |
| Height, cm | 177.1 (10.3) | 181.8 (6.2) | 180.7 (6.2) |
| Weight, kg | 84.1 (13.6) | 90.2 (15.7) | 89.3 (16.2) |
| CHD | 10 (8.7) | 27 (36.0) | 25 (33.8) |
| Hypertension | 56 (48.7) | 51 (68.0) | 44 (59.5) |
| CVD | 5 (4.4) | 18 (24.0) | 7 (9.5) |
| Claudication | 1 (0.9) | 8 (10.7) | 1 (1.4) |
| COPD | 5 (4.4) | 12 (16.0) | 11 (14.9) |
| Renal insufficiency | 2 (1.7) | 4 (5.3) | 4 (5.4) |
| Diabetes | 17 (14.8) | 17 (22.7) | 4 (5.4) |
| Aspirin use | 17 (14.8) | 37 (49.3) | 33 (44.6) |
| Statin use | 30 (26.1) | 35 (46.7) | 27 (36.5) |
| Current smoker | 6 (5.2) | 12 (16.0) | 25 (33.8) |
| Smoking years | 23.4 (13.7) | 34.2 (13.0) | 39.4 (14.3) |
Values are mean (SD) or n (%); D-dimer is missing in 4 participants.
CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
Circulating sGPVI and D-dimer are increased in patients with AAA and are predictive of AAA status and growth rate in the European cohort. (A-B) Plasma was assessed for sGPVI (A) and D-dimer (B) from healthy older control patients (n = 115), slow-growing AAAs (<2 mm/y; n = 75), or fast-growing AAAs (>2 mm/y; n = 74). The median (25th, 75th percentile) of sGPVI for controls was 14.9 ng/mL (8.7, 34.9), for slow-growing AAAs was 42.2 ng/mL (29.0, 62.7), and for fast-growing AAAs was 102.9 ng/mL (72.9, 132.9), represented by black bars (∗∗∗P < .001, by Kruskal-Wallis test). The median (25th, 75th percentile) of D-dimer for controls was 397.8 ng/mL (310.0, 525.8), for slow-growing AAAs was 779.5 ng/mL (508.7, 1184.9), and for fast-growing AAAs was 948.1 ng/mL (599.5, 1466.1), represented by black bars (∗∗∗P < .001, by Kruskal-Wallis test). (C) The OR and 95% CI for the association between sGPVI and case status (top) and D-dimer and case status (bottom). Both sGPVI and D-dimer were modeled dichotomously (above/below median) and continuously (log base-2 transformed). (D) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI (n = 149), D-dimer (n = 148), and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed, analysis restricted to cases only. (E) Comparative ROC curve analysis of sGPVI and D-dimer to distinguish fast- from slow-growing AAA using patients with slow-growing AAAs used as reference, (∗∗∗P < .001, as determined by ROC curve area comparison). (F) The effect of sGPVI adjusted for D-dimer (top) and D-dimer adjusted for sGPVI (middle) on case status was examined using ordinal logistic regression. The combined effect calculated using linear combination of sGPVI and D-dimer (bottom). (G) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI, D-dimer, and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed; analysis restricted to cases only (n = 148).
Circulating sGPVI and D-dimer are increased in patients with AAA and are predictive of AAA status and growth rate in the European cohort. (A-B) Plasma was assessed for sGPVI (A) and D-dimer (B) from healthy older control patients (n = 115), slow-growing AAAs (<2 mm/y; n = 75), or fast-growing AAAs (>2 mm/y; n = 74). The median (25th, 75th percentile) of sGPVI for controls was 14.9 ng/mL (8.7, 34.9), for slow-growing AAAs was 42.2 ng/mL (29.0, 62.7), and for fast-growing AAAs was 102.9 ng/mL (72.9, 132.9), represented by black bars (∗∗∗P < .001, by Kruskal-Wallis test). The median (25th, 75th percentile) of D-dimer for controls was 397.8 ng/mL (310.0, 525.8), for slow-growing AAAs was 779.5 ng/mL (508.7, 1184.9), and for fast-growing AAAs was 948.1 ng/mL (599.5, 1466.1), represented by black bars (∗∗∗P < .001, by Kruskal-Wallis test). (C) The OR and 95% CI for the association between sGPVI and case status (top) and D-dimer and case status (bottom). Both sGPVI and D-dimer were modeled dichotomously (above/below median) and continuously (log base-2 transformed). (D) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI (n = 149), D-dimer (n = 148), and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed, analysis restricted to cases only. (E) Comparative ROC curve analysis of sGPVI and D-dimer to distinguish fast- from slow-growing AAA using patients with slow-growing AAAs used as reference, (∗∗∗P < .001, as determined by ROC curve area comparison). (F) The effect of sGPVI adjusted for D-dimer (top) and D-dimer adjusted for sGPVI (middle) on case status was examined using ordinal logistic regression. The combined effect calculated using linear combination of sGPVI and D-dimer (bottom). (G) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI, D-dimer, and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed; analysis restricted to cases only (n = 148).
Linear regression modeling was applied to determine the association between sGPVI, D-dimer, and AAA growth rate. The mean AAA growth rate in patients with slow-growing AAAs was 0.8 mm/y (SD, 1.0), whereas fast-growing AAAs had a mean growth rate of 3.7 mm/y (SD, 1.6). After adjustment, per doubling of sGPVI, the β-coefficient for the growth rate was 1.6 (95% CI, 1.4-1.8; Figure 3D). Per doubling of D-dimer, the β-coefficient for the growth rate was lower in magnitude at 0.6 (95% CI, 0.3-0.9; Figure 3D) after adjustment than that for sGPVI. Moreover, in a comparative receiver operating characteristic (ROC) curve analysis of patients with AAA, plasma sGPVI concentration (n = 149) predicts the occurrence of fast-growing AAAs (area under the curve [AUC] = 0.81) with an optimum threshold of >64.5 ng/mL, with 77% specificity (95% CI, 66.7-85.3) and 81% sensitivity (95% CI, 70.7-88.4), significantly better than D-dimer concentrations (n = 148; AUC = 0.58) with an optimum threshold of >804 ng/mL with 56% specificity (95% CI: 44.8, 66.7) and 58% sensitivity (95% CI: 46.1, 68.2), when patients with slow-growing AAAs were used as control (Figure 3E).
To further understand the prognostic value of sGPVI and D-dimer, both ordinal logistic and linear regression models were reanalyzed with adjustments for sGPVI and D-dimer. Per doubling of sGPVI, when adjusted for covariates and D-dimer, the odds of being in the higher outcome group was 3.7 (95% CI, 2.6-5.2), whereas the odds ratio (OR) per doubling of D-dimer, adjusted for covariates and sGPVI, was 1.7 (95% CI, 1.1-2.4; Figure 3F). The combined effect of sGPVI and D-dimer on case status was significant after adjustment, with an OR of 1.8 (95% CI, 1.4-2.3; Figure 3F). After adjusting for comorbidities and D-dimer, per doubling of sGPVI, the β-coefficient for the growth rate was significant at 1.6 (95% CI, 1.4-1.9), whereas, after adjustment for sGPVI, the association between D-dimer and growth rate was no longer significant (Figure 3G). After adjustment, per doubling of sGPVI and D-dimer combined, the β-coefficient for the growth rate was 1.5 (95% CI, 1.3-1.8; Figure 3G).
We additionally examined the relationship between sGPVI, D-dimer, and AAA in participants from a separate American case-control study (Table 2). Concordant with the results in the European cohort, both plasma sGPVI and D-dimer were significantly increased in patients with AAA patients compared with control, with only sGPVI levels being significantly increased in patients with fast-growing vs slow-growing AAA (Figure 4A-B). Ordinal logistic regression demonstrates that, per doubling of sGPVI, the odds of being in a higher outcome group was 2.4 (95% CI, 1.9-3.0), and participants with sGPVI levels above the cohort median of 21 ng/mL had an OR of 5.2 (95% CI, 3.0-9.2) after adjustment (Figure 4C, top). In contrast to the European cohort, the effect of D-dimer per doubling (OR, 7.0; 95% CI, 4.4-10.9) was higher in magnitude than the effect of sGPVI. Results were similar in the American cohort for the associations between sGPVI, D-dimer, and growth rate, although in participants with D-dimer levels above 455 ng/mL, the association between D-dimer and growth rate was no longer significant (Figure 4D). Comparative ROC curve analysis using patients with AAA (n = 118) demonstrates that sGPVI (AUC = 0.85) with an optimum threshold of >51.8 ng/mL with 83% specificity (95% CI, 74.3-89.6) and 66% sensitivity (95% CI, 47.4-80.1) was significantly more predictive of fast-growing AAA than D-dimer (AUC = 0.58) with an optimum threshold of >852 ng/mL with 53% specificity (95% CI: 43.1, 63.3) and 79% sensitivity (95% CI: 61.6, 90.2) using patients with slow-growing AAA as control (Figure 4E).
Participant characteristics of the validation study by case definition (n = 218)
| Characteristics . | Control (n = 100) . | Slow-growing AAA (n = 89) . | Fast-growing AAA (n = 29) . |
|---|---|---|---|
| Age, y | 71.2 (8.4) | 71.8 (8.4) | 71.7 (8.1) |
| Sex | |||
| Male | 68 (68.0) | 72 (80.9) | 23 (79.3) |
| Female | 32 (32.0) | 17 (19.1) | 6 (20.7) |
| Race | |||
| White | 86 (86.0) | 84 (94.4) | 25 (86.2) |
| Other | 14 (14.0) | 5 (5.6) | 4 (13.8) |
| Height, in | — | 70 (67, 71) | 68 (65, 72) |
| Weight, kg | — | 83.5 (70.9, 95.7) | 82.6 (69.5, 102.5) |
| CAD | — | 42 (47.2) | 17 (58.6) |
| Hypertension | — | 76 (85.4) | 26 (89.7) |
| HLD | — | 76 (85.4) | 26 (89.7) |
| CKD | — | 19 (21.4) | 16 (55.2) |
| COPD | — | 32 (36.0) | 14 (48.3) |
| Diabetes | — | 25 (28.1) | 11 (37.9) |
| Aspirin use | — | 60 (67.4) | 20 (69.0) |
| Statin use | — | 65 (73.0) | 23 (79.3) |
| Current smoker | — | 26 (29.2) | 12 (41.4) |
| Growth, mm/y | — | 1 (0, 2) | 6 (5, 8) |
| Characteristics . | Control (n = 100) . | Slow-growing AAA (n = 89) . | Fast-growing AAA (n = 29) . |
|---|---|---|---|
| Age, y | 71.2 (8.4) | 71.8 (8.4) | 71.7 (8.1) |
| Sex | |||
| Male | 68 (68.0) | 72 (80.9) | 23 (79.3) |
| Female | 32 (32.0) | 17 (19.1) | 6 (20.7) |
| Race | |||
| White | 86 (86.0) | 84 (94.4) | 25 (86.2) |
| Other | 14 (14.0) | 5 (5.6) | 4 (13.8) |
| Height, in | — | 70 (67, 71) | 68 (65, 72) |
| Weight, kg | — | 83.5 (70.9, 95.7) | 82.6 (69.5, 102.5) |
| CAD | — | 42 (47.2) | 17 (58.6) |
| Hypertension | — | 76 (85.4) | 26 (89.7) |
| HLD | — | 76 (85.4) | 26 (89.7) |
| CKD | — | 19 (21.4) | 16 (55.2) |
| COPD | — | 32 (36.0) | 14 (48.3) |
| Diabetes | — | 25 (28.1) | 11 (37.9) |
| Aspirin use | — | 60 (67.4) | 20 (69.0) |
| Statin use | — | 65 (73.0) | 23 (79.3) |
| Current smoker | — | 26 (29.2) | 12 (41.4) |
| Growth, mm/y | — | 1 (0, 2) | 6 (5, 8) |
Values are presented as mean (SD) or n (%).
CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HLD, hyperlipidemia.
Circulating sGPVI and D-dimer are elevated in patients with AAA and are predictive for case status and growth rate in the American case-control study. (A-B) Plasma was assessed for sGPVI (A) and D-dimer (B) from healthy older control patients (n = 100), slow-growing AAAs (<4 mm/y; n = 89), or fast-growing AAAs (>4 mm/y; n = 29). The median (25th, 75th percentile) of sGPVI for controls was 15.8 ng/mL (10.8, 23.2), for slow-growing AAAs was 20.9 ng/mL (14.3, 35.2), and for fast-growing AAAs was 138.2 ng/mL (39.2, 176.4), represented by black bars (∗∗∗P < .001, by Kruskal-Wallis test). The median (25th, 75th percentile) of D-dimer for controls was 256 ng/mL (184, 340), for slow-growing AAAs was 795 ng/mL (551, 1139), and for fast-growing AAAs was 1123 ng/mL (854, 1633), represented by black bars (∗∗P = .002; ∗∗∗P < .001, by Kruskal-Wallis test). (C) The OR and 95% CI for the association between sGPVI and case status (top) and D-dimer and case status (bottom). Both sGPVI and D-dimer were modeled dichotomously (above/below median) and continuously (log base-2 transformed). (D) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI (n = 118), D-dimer (n = 118), and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed; analysis restricted to cases only. (E) Comparative ROC analysis using sGPVI and D-dimer to predict the occurrence of fast-growing AAAs using patients with slow-growing AAAs used as reference (∗P = .04, as determined by ROC curve area comparison). (F) The effect of sGPVI adjusted for D-dimer (top) and D-dimer adjusted for sGPVI (middle) on case status was examined using ordinal logistic regression. The combined effect calculated using linear combination of sGPVI and D-dimer (bottom). (G) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI, D-dimer, and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed; analysis restricted to cases only (n = 118).
Circulating sGPVI and D-dimer are elevated in patients with AAA and are predictive for case status and growth rate in the American case-control study. (A-B) Plasma was assessed for sGPVI (A) and D-dimer (B) from healthy older control patients (n = 100), slow-growing AAAs (<4 mm/y; n = 89), or fast-growing AAAs (>4 mm/y; n = 29). The median (25th, 75th percentile) of sGPVI for controls was 15.8 ng/mL (10.8, 23.2), for slow-growing AAAs was 20.9 ng/mL (14.3, 35.2), and for fast-growing AAAs was 138.2 ng/mL (39.2, 176.4), represented by black bars (∗∗∗P < .001, by Kruskal-Wallis test). The median (25th, 75th percentile) of D-dimer for controls was 256 ng/mL (184, 340), for slow-growing AAAs was 795 ng/mL (551, 1139), and for fast-growing AAAs was 1123 ng/mL (854, 1633), represented by black bars (∗∗P = .002; ∗∗∗P < .001, by Kruskal-Wallis test). (C) The OR and 95% CI for the association between sGPVI and case status (top) and D-dimer and case status (bottom). Both sGPVI and D-dimer were modeled dichotomously (above/below median) and continuously (log base-2 transformed). (D) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI (n = 118), D-dimer (n = 118), and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed; analysis restricted to cases only. (E) Comparative ROC analysis using sGPVI and D-dimer to predict the occurrence of fast-growing AAAs using patients with slow-growing AAAs used as reference (∗P = .04, as determined by ROC curve area comparison). (F) The effect of sGPVI adjusted for D-dimer (top) and D-dimer adjusted for sGPVI (middle) on case status was examined using ordinal logistic regression. The combined effect calculated using linear combination of sGPVI and D-dimer (bottom). (G) Unadjusted (blue) and adjusted (red) linear regression for the relationship between sGPVI, D-dimer, and the growth rate (mm/year) of AAA. Continuous sGPVI and D-dimer are log transformed; analysis restricted to cases only (n = 118).
The combined effects of sGPVI and D-dimer on case status and growth rate for the American case-control study are presented in Figure 4F. In contrast with the results in the European cohort, the effect of D-dimer (OR, 7.0; 95% CI, 4.4-10.9) was higher in magnitude than the effect of sGPVI (OR, 1.3; 95% CI, 0.9-1.7) on case status in the American cohort. Interestingly, after adjustment for sGPVI, D-dimer was not associated with growth rate (β-coefficient, 0.4; 95% CI, –0.8 to 1.6), whereas sGPVI, after adjustment for D-dimer, had a significant association with growth rate (β-coefficient, 1.2; 95% CI, 0.5-1.9; Figure 4G). Taken together, these results suggest that both sGPVI and D-dimer can assist in AAA diagnosis, whereas sGPVI is more informative with respect to predicting an accelerated rate of AAA growth.
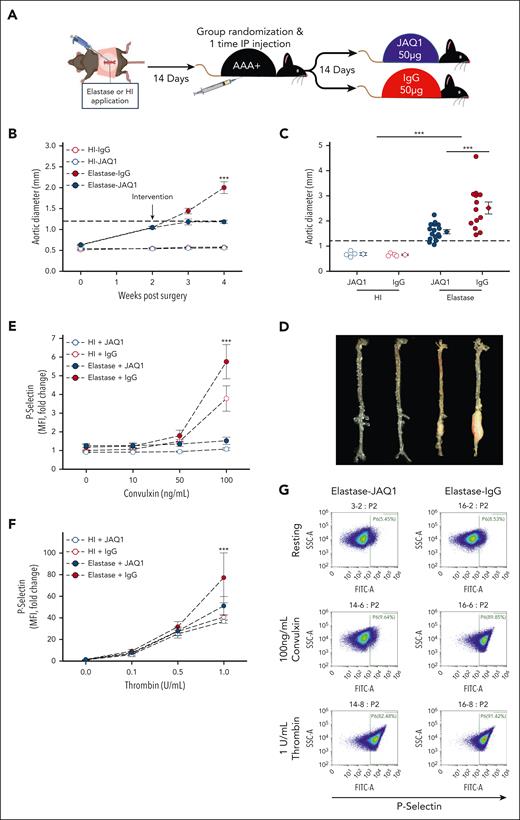
Platelet targeting via GPVI inhibition via JAQ1 antibody intervention blunts progression of established AAAs in murine models
In mice, JAQ1 antibody injection inhibits GPVI-mediated platelet activation with minimal effect on bleeding times.30 To test the efficacy of targeting GPVI to reduce AAA burden, male C57BL/6J mice were subjected to open laparotomy, and either topical elastase (10 mg/mL for 5 minutes) or heat inactivated (HI) elastase was applied to the infrarenal aorta. Next, 14 days after laparotomy, mice were blindly randomized into 4 groups for intervention with the JAQ1 antibody or rat IgG2a control (50 μg; Figure 5A): HI + IgG (n = 4), HI + JAQ1 (n = 4), elastase + IgG (n = 15), and elastase + JAQ1 (n = 16). Aortic diameter was tracked by ultrasound, and groups with similar AAA status (HI vs elastase) were normalized for aortic diameter prior to randomization (Figure 5B). JAQ1 antibody injection did not affect body weight (supplemental Figure 3A). Mice in the elastase group treated with JAQ1 had significantly less aortic growth and a lower final aortic diameter after intervention than control (Figure 5B-D), and no mice treated with JAQ1 experienced rupture, whereas 2 of 15 mice in the control group died from aortic rupture (supplemental Figure 3B). JAQ1-treated mice had significantly more type I collagen (supplemental Figure 3C left, D), however, no difference in macrophage accumulation was detected (supplemental Figure 3C right, E).
GPVI blockade via intervention with JAQ1 antibody mitigates elastase AAA expansion. (A) Study schematic. Male Ldlr−/− mice (aged 8-10 weeks) underwent laparotomy and elastase (5 μL of 10 mg/mL porcine pancreatic elastase; closed circles) or HI elastase (open circles) as sham was applied to the adventitia of infrarenal aorta for 5 minutes to initiate AAA. At 14 days after laparotomy, mice were blindly randomized while normalized for aortic diameter and given either a 1-time IP injection of 50-μg JAQ1 antibody (n = 16, blue) or 50-μg IgG control (n = 13, red). After another 14 days, mice were euthanized, final aortic diameter was measured ex vivo, and tissues were harvested for further analysis. (B) Intraluminal aortic diameters measured by weekly ultrasound (∗∗∗P < .001, elastase-IgG vs elastase-JAQ1 by 3-way ANOVA with Holm-Sidak post hoc analysis). (C) Aortic diameters in JAQ1-treated vs control-treated mice measured ex vivo 28 days after elastase surgery (∗∗∗P < .001, elastase vs HI and elastase-IgG vs elastase-JAQ1 by 2-way ANOVA with Holm-Sidak post hoc analysis). (D) Representative images of aortas ex vivo from the indicated groups. Platelet activation measured 14 days after IgG or JAQ1 antibody intervention indicated by surface P-selectin (FITC) levels in response to varying doses of the GPVI specific agonist convulxin (E) or thrombin (F) in JAQ1 vs IgG-treated mice measured by flow cytometry (n = 4-6 per group). As expected, a significant reduction in convulxin-mediated (E) activation was observed in platelets isolated from mice treated with JAQ1 antibody (P < .001, for the interaction between intervention and platelet treatment assessed by 3-way ANOVA; ∗∗∗P < .001, between JAQ1 and IgG by Holm-Sidak post hoc analysis). (F) In response to thrombin, a significant increase in platelet activation was observed in mice with a AAA (closed circles) compared with mice without a AAA (open circles) (P = .024, for the interaction between AAA status and platelet treatment assessed by 3-way ANOVA; ∗∗∗P <.001, for comparison of surgery within 1 U/mL thrombin treatment by Holm-Sidak post hoc analysis). No significant effect of JAQ1 intervention on thrombin mediate platelet activation was noted (P = .140, for the interaction between intervention and platelet treatment). (G) Representative flow cytometry scatterplots from the indicated conditions. Data are represented as individual data points or mean ± SEM. FITC, fluorescein isothiocyanate; IP, intraperitoneal.
GPVI blockade via intervention with JAQ1 antibody mitigates elastase AAA expansion. (A) Study schematic. Male Ldlr−/− mice (aged 8-10 weeks) underwent laparotomy and elastase (5 μL of 10 mg/mL porcine pancreatic elastase; closed circles) or HI elastase (open circles) as sham was applied to the adventitia of infrarenal aorta for 5 minutes to initiate AAA. At 14 days after laparotomy, mice were blindly randomized while normalized for aortic diameter and given either a 1-time IP injection of 50-μg JAQ1 antibody (n = 16, blue) or 50-μg IgG control (n = 13, red). After another 14 days, mice were euthanized, final aortic diameter was measured ex vivo, and tissues were harvested for further analysis. (B) Intraluminal aortic diameters measured by weekly ultrasound (∗∗∗P < .001, elastase-IgG vs elastase-JAQ1 by 3-way ANOVA with Holm-Sidak post hoc analysis). (C) Aortic diameters in JAQ1-treated vs control-treated mice measured ex vivo 28 days after elastase surgery (∗∗∗P < .001, elastase vs HI and elastase-IgG vs elastase-JAQ1 by 2-way ANOVA with Holm-Sidak post hoc analysis). (D) Representative images of aortas ex vivo from the indicated groups. Platelet activation measured 14 days after IgG or JAQ1 antibody intervention indicated by surface P-selectin (FITC) levels in response to varying doses of the GPVI specific agonist convulxin (E) or thrombin (F) in JAQ1 vs IgG-treated mice measured by flow cytometry (n = 4-6 per group). As expected, a significant reduction in convulxin-mediated (E) activation was observed in platelets isolated from mice treated with JAQ1 antibody (P < .001, for the interaction between intervention and platelet treatment assessed by 3-way ANOVA; ∗∗∗P < .001, between JAQ1 and IgG by Holm-Sidak post hoc analysis). (F) In response to thrombin, a significant increase in platelet activation was observed in mice with a AAA (closed circles) compared with mice without a AAA (open circles) (P = .024, for the interaction between AAA status and platelet treatment assessed by 3-way ANOVA; ∗∗∗P <.001, for comparison of surgery within 1 U/mL thrombin treatment by Holm-Sidak post hoc analysis). No significant effect of JAQ1 intervention on thrombin mediate platelet activation was noted (P = .140, for the interaction between intervention and platelet treatment). (G) Representative flow cytometry scatterplots from the indicated conditions. Data are represented as individual data points or mean ± SEM. FITC, fluorescein isothiocyanate; IP, intraperitoneal.
To confirm that JAQ1 treatment resulted in GPVI blockade, washed platelets were prepared from mice treated with HI + IgG (n = 4), HI + JAQ1 (n = 4), elastase + IgG (n = 4), and elastase + JAQ1 (n = 6) 28 days after laparotomy. Interestingly, AAA status was associated with an increase in platelet reactivity upon either convulxin or thrombin stimulation (HI vs elastase; Figure 5E-F). As expected, IgG-treated platelets activated in a dose dependent manner to both convulxin (GPVI agonist) or thrombin (murine platelet PAR3 and PAR4 agonist)48 stimulation (Figure 5E-F). Although platelets from elastase + JAQ1–treated mice were less hyperactive than platelets from IgG control–treated mice when exposed to thrombin, convulxin treatment elicited no activation, indicating successful blockade of the platelet GPVI receptor (Figure 5E-F). Finally, although a mild transient drop in platelet count was observed with JAQ1 treatment as previously reported (supplemental Figure 3F),30 platelet counts normalized by day 5, and there was no difference in platelet count between groups at study conclusion (supplemental Figure 3G).
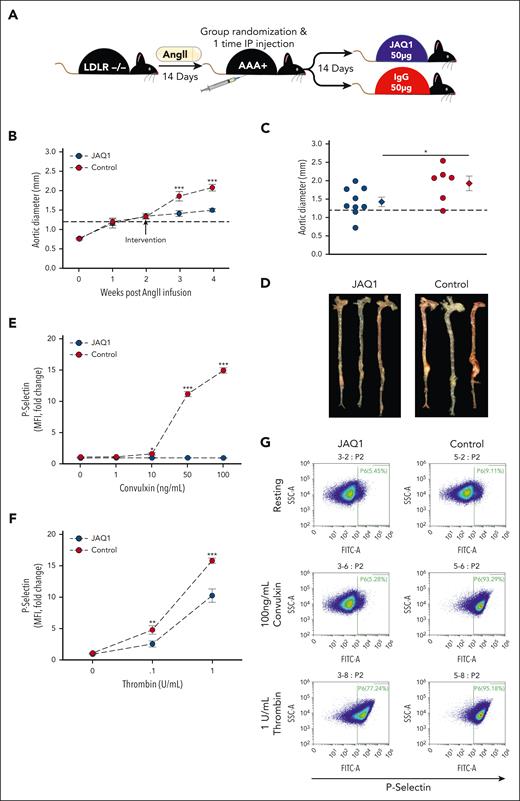
As a second model of AAA, male Ldlr−/− underwent AngII infusion for 28 days. Blinded randomization into JAQ1 (n = 9) and control (n = 9) intervention groups with equal aortic diameter was performed on day 14 (Figure 6A). Mice that received JAQ1 antibody demonstrated significantly less aortic growth post intervention compared with control-treated mice (Figure 6B). Moreover, JAQ1-treated mice experienced zero rupture-induced deaths, whereas 3 of 9 mice in the control group died from aortic rupture (supplemental Figure 4B). Final aortic diameter was assessed ex vivo with JAQ1-treated mice having significantly smaller aortic diameters than control mice (Figure 6C-D). Additionally, JAQ1-treated mice had significantly more type I collagen, as measured by picrosirius red (supplemental Figure 4C-D), and less CD68 infiltrating macrophages (supplemental Figure 4C,E) than control-treated mice. Finally, treatment of washed platelets with convulxin and thrombin demonstrated that JAQ1 intervention specifically inhibited GPVI-mediated platelet activation (Figure 6E-G). The congruent results obtained in 2 murine AAA models suggest platelet inhibition by selective GPVI targeting is a feasible therapeutic option to limit AAA progression.
GPVI blockade via intervention with JAQ1 antibody blunts AngII–induced AAA expansion and rupture-induced death. (A) Study schematic. Male Ldlr−/− mice (aged 8-10 weeks) were infused with AngII (1000 ng/kg per day) via osmotic pump implantation. After 14 days of AngII infusion, mice were blindly randomized into 2 groups of controlled for aortic diameter and given either a 1-time IP injection of 50-μg JAQ1 antibody (n = 9) or 50-μg IgG control (n = 9). After another 14 days, mice were euthanized, final aortic diameter was measured ex vivo, and tissues were harvested for further analysis. (B) Intraluminal aortic diameters measured by weekly ultrasound (∗∗∗P < .001, JAQ1 vs control by 2-way repeated measures ANOVA). (C) Aortic diameters in JAQ1-treated vs control-treated mice measured ex vivo 28 days after AngII infusion (∗P = .045 by Student t test). (D) Representative images of aortas ex vivo from the indicated groups. Platelet activation measured 14 days after JAQ1 or IgG antibody intervention indicated by surface P-selectin (FITC) levels in response to varying doses of the GPVI specific agonist convulxin (E) or thrombin (F) in JAQ1-treated vs control mice measured flow cytometry (n = 6 per group; ∗P = .032; ∗∗P = .01; ∗∗∗P < .001, JAQ1 vs control by 2-way ANOVA). (G) Representative flow cytometry scatterplots from the indicated conditions. Data are represented as individual data points or mean ± SEM. IP, intraperitoneal.
GPVI blockade via intervention with JAQ1 antibody blunts AngII–induced AAA expansion and rupture-induced death. (A) Study schematic. Male Ldlr−/− mice (aged 8-10 weeks) were infused with AngII (1000 ng/kg per day) via osmotic pump implantation. After 14 days of AngII infusion, mice were blindly randomized into 2 groups of controlled for aortic diameter and given either a 1-time IP injection of 50-μg JAQ1 antibody (n = 9) or 50-μg IgG control (n = 9). After another 14 days, mice were euthanized, final aortic diameter was measured ex vivo, and tissues were harvested for further analysis. (B) Intraluminal aortic diameters measured by weekly ultrasound (∗∗∗P < .001, JAQ1 vs control by 2-way repeated measures ANOVA). (C) Aortic diameters in JAQ1-treated vs control-treated mice measured ex vivo 28 days after AngII infusion (∗P = .045 by Student t test). (D) Representative images of aortas ex vivo from the indicated groups. Platelet activation measured 14 days after JAQ1 or IgG antibody intervention indicated by surface P-selectin (FITC) levels in response to varying doses of the GPVI specific agonist convulxin (E) or thrombin (F) in JAQ1-treated vs control mice measured flow cytometry (n = 6 per group; ∗P = .032; ∗∗P = .01; ∗∗∗P < .001, JAQ1 vs control by 2-way ANOVA). (G) Representative flow cytometry scatterplots from the indicated conditions. Data are represented as individual data points or mean ± SEM. IP, intraperitoneal.
Discussion
This study revealed GPVI as the first potential target (to our knowledge) offering both diagnostic and therapeutic potential in patients with infrarenal aortic aneurysmal disease, conducted in both humans with AAA and in 2 independent murine models of AAA. The latest guidelines issued jointly by the American Heart Association and the American College of Cardiology give the platelet inhibitor aspirin a class IIb recommendation in managing patients with AAA with ILT or penetrating aortic ulcer but also with the presumption of concomitant arterial atherosclerosis.49 However, this recommendation was made by expert consensus rather than the availability of mechanistic data.49 Our data demonstrate that GPVI is enriched in AAA-associated ILTs, functionally increased in patients with AAA, and sGPVI is positively associated with an aneurysmal infrarenal aorta as well as aortic aneurysm growth rate. This observation was fully reproducible in 2 independent human cohorts evaluated in 2 different countries. Of note, aspirin use did not appear to directly influence sGPVI because there was no significant difference between no aspirin use and aspirin use when comparing sGPVI levels in control or patients with AAA as individual groups (data not shown). Additionally, comparing sGPVI with D-dimer (the strongest biomarker to date for predicting adverse AAA outcomes), sGPVI was demonstrated to better predict AAA progression in patients with established infrarenal aortic aneurysmal disease. Taken together, our data indicate that although D-dimer and sGPVI correlate within individual patients (supplemental Figure 2A-B) and similarly predict a diagnosis of AAA, sGPVI concentration better identifies patients with fast AAA growth and reveals itself to be a useful biomarker for clinical decision-making.
The size and growth rate of ILT directly correlate with the risk of AAA rupture and exist in a balance of active thrombus generation and resolution, although total resolution is rarely achieved.13,50 As the most robust blood biomarker of platelet activation, elevated sGPVI suggests platelet adhesion and deposition at the luminal surface of the ILT, which is a dynamic and progressive process that may promote AAA expansion. Conversely, D-dimer is a product of clot resolution, and although levels in circulation are highly sensitive for detecting intravascular coagulation and clot breakdown, it is not a direct measure of thrombus formation.51 Although D-dimer undoubtedly indicates the presence of ILT associated with AAA, our data indicate that sGPVI is a more sensitive marker of ongoing ILT formation and active AAA growth. Therefore, we conclude that measuring sGPVI concentration in patients with AAA, especially in newly identified patients, will improve the identification of those at the greatest risk to experience AAA rupture to help clinicians make decisions on frequency of follow-up monitoring and when to intervene surgically.
A strength of this study is the use of 2 independent patient cohorts from distinct geographic regions that favors generalizability of the scientific observations and allows us to compare separate populations with different modifiable risk factors. Although hypertension and smoker status were prevalent in the European cohort, the American cohort had more comorbidities including chronic obstructive pulmonary disease and diabetes. Additionally, the American cohort included women (Table 2), a population with a lower incidence of AAA, but an increased preponderance for unpredictable ruptures occurring at smaller abdominal diameters.4,52,53 Control patient covariates including height, weight, smoking, comorbidities, medication use, and aorta diameter were not available for the American cohort, which is a limitation. Female patients of the European cohort were excluded because no appropriate controls were available. Another strength is the use of a population-based case-control study design for both cohorts and the relatively large sample size of slow- and fast-growing AAAs in the European cohort. Using both cohorts, we were able to reanalyze and directly compare our previously published results on the association of D-dimer,8 the most established biomarker for AAA, with our novel analysis of sGPVI. Although aortic diameter was assessed longitudinally in both cohorts, no further follow-up data were available regarding rupture or surgical intervention because these patients were excluded. Subsequently, further prospective trials including longitudinal plasma sampling are needed to define clinical thresholds for D-dimer and sGPVI as biomarkers of AAA status and growth rate.
We have identified a role for platelet activation in the progression of vascular degeneration and rupture in a mouse model of AAA.2,54 Recent work from our collective group demonstrates a mechanistic link between increased platelet reactivity and AAA progression with concomitant increases in platelet receptor expression.55 This suggests that the circulating platelet in AAA has a divergent phenotype from healthy conditions and may offer diagnostic as well as therapeutic potential. Studies targeting platelets with GPIIb/IIIa or P2Y12 (purinergic receptor P2Y, G-protein coupled, 12 protein) inhibitors successfully decreased AAA burden in rodent models,2,56,57 however, results from clinical data with respect to antiplatelet drugs’ effects on AAA outcome in humans are mixed.7,29 Current platelet inhibitors are associated with bleeding, limiting their utility in patients with AAA, creating a need for the development of alternative antiplatelet targets. GPVI antagonism provides a favorable approach to limit pathologic platelet aggregation without bleeding.21,30,58-61 The minimal bleeding phenotype associated with loss of GPVI is attributed to the existence of several redundant collagen-responsive platelet activation pathways including integrin α2β1 and GPV receptors, as well as GPIbα and αIIβ3 interaction with collagen-bound von Willebrand factor.21,62-64 Although this suggests that platelet-mediated primary hemostasis is not dependent on GPVI activity, GPVI is critical to platelet adhesion, propagation, and stability of an existing thrombus via efficient phosphorylation of secondary messengers, robust release of Ca2+ and adenosine diphosphate, and increased activity of integrins in response to collagen binding.18-21,65,66 Moreover, GPVI binding of fibrin facilitates platelet activation, stable platelet adhesion, and platelet spreading with concomitant augmentation of thrombin generation via increased phosphatidylserine exposure.18,21,67 The accumulation of activated platelets at the luminal surface of an aneurysm contributes to the deposition of new ILT layers and overall AAA growth rate, which has been linked to fatal aortic rupture.5,13 Therefore, we postulate that the blockade of GPVI effectively reduces platelet aggregation on the exposed collagen and fibrin matrix present at the site of ILT and in turn blunts AAA progression.
Due to the appeal of targeting platelets to treat cardiovascular disease without affecting primary hemostasis, antagonists of GPVI have been developed and brought into clinical trials. Revacept is a recombinant competitive inhibitory protein ligand for collagen binding to GPVI, thus reducing platelet activation, as seen in experimental models of thrombosis.68,69 Phase 2 clinical trials show Revacept reduces ischemic lesions in symptomatic patients with internal carotid artery stenosis,70 however, myocardial injury was unchanged between Revacept and placebo in stable patients with ischemic heart disease undergoing percutaneous intervention.71 Although exposed collagen is abundant at the luminal site of AAA, it is structurally abnormal, which may limit the effectiveness of Revacept in patients with AAA.72 A second clinically relevant compound is glenzocimab, which directly binds to GPVI, blocking the interaction with collagen and fibrinogen/fibrin and decreasing platelet adhesion and thrombosis. Glenzocimab combined with recombinant tissue plasminogen activator attenuated symptomatic intracerebral hemorrhage, a condition associated with altered GPVI activity and expression, and improves the survival of patients.73 Additionally, glenzocimab treatment is associated with thrombus disintegration, which could aid in the regression of AAA-associated ILTs and improve patient outcomes. Conversely, destabilization of a large ILT could cause thromboembolism and increase the risk of myocardial infarction and stroke, as noted in patients with endovascular aneurysm repair.74 Importantly, increased bleeding times have not been observed with Revacept or glenzocimab, and both are well tolerated by patients.35,67 Yet, these compounds have not been tested in patients with AAA. Given that the risk of adverse bleeding events and AAA incidence increases with age, it is vital to develop therapeutics that reduce AAA burden without bleeding side effects. Targeting GPVI with antagonists in clinical development presents an exciting opportunity as the first viable therapeutic intervention for patients with AAA, but more work is needed to fully understand the implications of such a strategy in aneurysmal pathogenesis.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (grants R01-HL147171 [A.P.O. III]; R01-HL158801 [S.J.C.]; F32 HL172601 [T.W.B.]; and T32 HL125204 [A.S.]), the Swedish Research Council (grants K2013-64X-20406-07-3 and K2013-99X-22275-01-3 [A.W. and M.B.]), the Swedish Heart-Lung Foundation (grants 2012-0353 and 2015-0596 [A.W. and M.B.]), and the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse (A.W.). This work was also supported by funding from the National Health and Medical Research Council of Australia (E.E.G.); and Clinical Translational Science Award (TL1TR002244) from the National Center for Advancing Translational Sciences (M.M.P.). Aspects of the project were supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, award number 2UL1TR001425.
Authorship
Contribution: T.W.B., A.S., H.M.R., S.J.C, N.M., and A.P.O. III conceived and designed the study; T.W.B., A.S., H.M.R., T.M.C, S.I.B., and C.W.-L. were responsible for animal care; S.J.C., R.B., S.S., D.S.M., C.J., F.J.C., O.Q., S.L., A.A., K.M., M.B., A.W., and M.P. were responsible for phlebotomy, clinical data management, and regulation; M.M.P., L.L.-E, C.R.-C., T.L.E., and M.T. performed statistical analysis of human cohort data; T.W.B., S.A., K.A.C., S.J.C., A.A., S.M.H., E.E.G., R.B., A.S., H.M.R., T.M.C, S.I.B., C.W.-L., B.T., and A.P.O. III conducted laboratory testing; T.W.B., A.S., H.M.R., M.M.P., S.S., M.P., and A.P.O. III contributed to data processing; T.W.B., A.S., and A.P.O. III supervised all aspects of the study; T.W.B., A.S., M.M.P., and A.P.O. III contributed to initial data interpretation and wrote the manuscript; and all authors contributed to final data interpretation and critical revision of the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Phillip Owens III, University of Cincinnati, 231 Albert Sabin Way ML: 0542, Cincinnati, OH 45267-0542; email: phillip.owens@uc.edu.
References
Author notes
T.W.B., M.M.P., and A.S. are joint first authors and contributed equally to this study.
Our RNA sequencing data have been deposited in National Center for Biotechnology Information Gene Expression Omnibus database (accession number GSE269845).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal