Visual Abstract
Rare subtypes of peripheral T-cell lymphoma (PTCL) including enteropathy-associated T-cell lymphoma (EATL), monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), subcutaneous panniculitis–like T-cell lymphoma (SPTCL), and hepatosplenic T-cell lymphoma (HSTCL) are underrepresented in most registries and clinical studies. Most of the literature is obtained from small case series, single-institution retrospective studies, and subgroup analyses of the largest studies with few recent and ongoing exceptions. Although the pathogenesis and biology of these entities have yet to be fully elucidated, global efforts by the scientific community have started to shed some light on the most frequently deregulated pathways. In this review, we highlight the most pertinent clinical and pathologic features of rare subtypes of PTCL including EATL/MEITL, SPTCL, and HSTCL. We also summarize the results of recent developments identifying potential targets for novel therapeutic strategies based on molecular studies. Finally, we highlight the underrepresentation of these rare subtypes in most clinical trials, making evidence–based therapeutic decisions extremely challenging.
Introduction
The International T Cell Lymphoma Project reported a retrospective analysis of 1314 cases of mature T-cell lymphomas from 22 worldwide centers collected from 1990 to 2002. This study1 and others2 reported enteropathy-associated T-cell lymphoma (EATL) to comprise 4.7% of all cases, subcutaneous panniculitis-like T-cell lymphoma (SPTCL) 0.9%, and hepatosplenic T-cell lymphoma (HSTCL) 1.4%, for a total of about 90 patients with these 3 rare subtypes. More recently, these data were updated by the T-cell Project, which described the incidence and outcome of rare T-cell lymphomas collected since 2006 and showed a similar relative frequency of EATL at 2% (31/1553 cases), SPTCL 1.5% (65/1553), and HSTCL 2% (31/1553), summing up to a total of 120 cases collected in 14 years.3 Of note, in these analyses, all subtypes of intestinal T-cell lymphoma were incorporated within the EATL subgroup. These data emphasize the major challenge with these entities, which is related to their extreme rarity.
The modern classification of these lesions is the result of advances in laboratory testing and improved understanding of their respective biologies and natural histories. Despite the challenges imposed by evolving nomenclature and limited prior data, the recognition of key signaling pathways and genetic traits provides ample opportunity for strategic intervention. To this end, we also summarize the results of recent developments identifying potential targets for novel therapeutic strategies based on molecular studies. Finally, we emphasize the underrepresentation of these rare subtypes in most clinical trials exploring the value of first-line treatment and the activity of novel agents in peripheral T-cell lymphoma (PTCL) (Table 1) as a major hurdle that must be overcome to establish evidence–based standard of care.
Overview of important clinical studies in first line for PTCL and the representation of rare PTCL subtypes
| Investigational agent used . | Total number of patients . | EATL/MEITL, n (best response) . | SPTCL n (best response) . | HSTCL n (best response) . | Responses in other/unspecified subtypes in publication . |
|---|---|---|---|---|---|
| Brentuximab + CHP followed by ASCT (EATL-001, Sibon et al)49 | 14 | 14 (ORR = 79%, CR = 64%, 2-y PFS = 63%, 2-y OS = 68%) | 0 (0) | 0 (0) | NA |
| Brentuximab + CHP followed by ASCT (ECHELON-2, Horwitz et al)106 | 452 | 3 (0) | 0 (0) | 0 (0) | |
| Romidepsin + CHOP vs CHOP (Bachy et al)107 | 421 | 16 (5 Ro-CHOP, 11 CHOP) (NR) | 9 (7 Ro-CHOP, 2 CHOP) (NR) | 2 (1 Ro-CHOP, 1 CHOP) | No significant difference in PFS between Ro-CHOP and CHOP for “other” PTCL histologies |
| CHOP/ICE/IVAC followed by Auto/Allo (Voss et al)99 | 14 | 0 (0) | 0 (0) | 14 (ORR =64%, CR = 35%, 5-y OS = 50%) | NA |
| CHOEP followed by Auto/Allo (Schmitz et al)54 | 103 | 3 (NR) | 1 (NR) | 2 (NR) | 1 primary cutaneous γ/δ T-cell lymphoma (outcome NR) |
| CHEOP followed by ASCT (NLG-T-01, d’Amore et al)46 | 160 | 21 (5-y PFS = 38%, 5-y OS = 48%) | 6 (3-y OS = 44%) ∗3-y OS for SPTCL and HSTCL estimated as composite of all patients with SPTCL, HSTCL, and ENKTL | 5 (3-y OS = 44%) ∗3-y OS for SPTCL and HSTCL estimated as composite of all patients with SPTCL, HSTCL, and ENKTL | 5 patients with ENKTL |
| Investigational agent used . | Total number of patients . | EATL/MEITL, n (best response) . | SPTCL n (best response) . | HSTCL n (best response) . | Responses in other/unspecified subtypes in publication . |
|---|---|---|---|---|---|
| Brentuximab + CHP followed by ASCT (EATL-001, Sibon et al)49 | 14 | 14 (ORR = 79%, CR = 64%, 2-y PFS = 63%, 2-y OS = 68%) | 0 (0) | 0 (0) | NA |
| Brentuximab + CHP followed by ASCT (ECHELON-2, Horwitz et al)106 | 452 | 3 (0) | 0 (0) | 0 (0) | |
| Romidepsin + CHOP vs CHOP (Bachy et al)107 | 421 | 16 (5 Ro-CHOP, 11 CHOP) (NR) | 9 (7 Ro-CHOP, 2 CHOP) (NR) | 2 (1 Ro-CHOP, 1 CHOP) | No significant difference in PFS between Ro-CHOP and CHOP for “other” PTCL histologies |
| CHOP/ICE/IVAC followed by Auto/Allo (Voss et al)99 | 14 | 0 (0) | 0 (0) | 14 (ORR =64%, CR = 35%, 5-y OS = 50%) | NA |
| CHOEP followed by Auto/Allo (Schmitz et al)54 | 103 | 3 (NR) | 1 (NR) | 2 (NR) | 1 primary cutaneous γ/δ T-cell lymphoma (outcome NR) |
| CHEOP followed by ASCT (NLG-T-01, d’Amore et al)46 | 160 | 21 (5-y PFS = 38%, 5-y OS = 48%) | 6 (3-y OS = 44%) ∗3-y OS for SPTCL and HSTCL estimated as composite of all patients with SPTCL, HSTCL, and ENKTL | 5 (3-y OS = 44%) ∗3-y OS for SPTCL and HSTCL estimated as composite of all patients with SPTCL, HSTCL, and ENKTL | 5 patients with ENKTL |
ASCT, autologous stem cell transplant; CAR, chimeric antigen receptor; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; ICE, ifosfamide, carboplatin, etoposide; NA, not applicable; NR, not reported; SD, stable disease.
EATL and MEITL
Epidemiology
EATL and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) are rare and aggressive subtypes of PTCL that primarily affect the small intestine. Their current definitions and nomenclature were established in 20164 and remain unchanged in both the World Health Organization (WHO) fifth classification of hematolymphoid tumors5 and International Consensus Classification (ICC) of mature lymphoid neoplasms.6 EATL (previously type I EATL) is frequently associated with celiac disease and observed in Northern Europe, whereas MEITL (previously type II EATL) occurs de novo and is predominantly diagnosed in Asian populations.7,8
Clinical presentation and diagnosis
EATL is derived from the malignant transformation of intestinal intraepithelial lymphocytes and is considered the most common neoplastic complication of celiac disease,9 with a lifetime incidence of ∼1% to 2% in these patients and male predominance. EATL is more frequently observed in Europe (0.05-0.14/100 000)10,11 and the United States (0.016/100 000),12 where the celiac risk alleles HLA DQA1∗0501 and DQB1∗020113 are more common. Homozygosity for HLA-DQ2.5, poor adherence to a gluten-free diet, and old age are risk factors for EATL.10,13 EATL may be preceded by refractory celiac disease, defined as persistent or recurrent symptoms and signs of malabsorption with villous atrophy despite a strict gluten-free diet for >12 months. Currently, refractory celiac disease is categorized into 2 types, based on immunophenotypic and molecular criteria. In refractory celiac disease type II, intraepithelial lymphocytes exhibit an aberrant immunophenotype along with a high frequency of clonal T-cell receptor (TCR) gene rearrangements and/or somatic alterations overlapping with those found in de novo EATL (eg, recurrent JAK-STAT pathway mutations).14,15 In contrast, the intraepithelial lymphocytes in refractory celiac disease type I exhibit a normal immunophenotype (positive for surface CD3 and CD8) and are typically polyclonal. Refractory celiac disease type II is currently recognized by both the ICC and WHO as a premalignant condition associated with high rates of transformation to EATL.6 Per American Gastroenterological Association Clinical Practice Guidelines, corticosteroids are the medication of choice and should be used as first-line therapy in all forms of refractory celiac disease.16
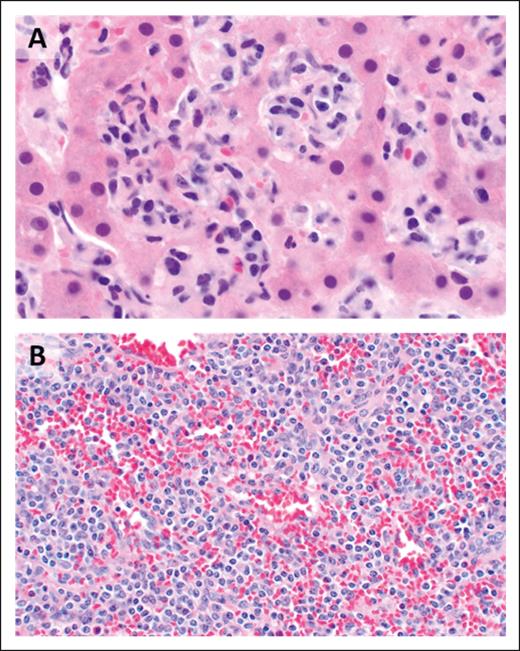
The histopathologic features of EATLs are highly variable. The neoplastic lymphocytes are generally medium to large in size and show varying degrees of pleomorphism, with some cases displaying immunoblastic or anaplastic cytomorphology.17 The tumors are frequently accompanied by a mixed inflammatory infiltrate composed of histiocytes, eosinophils, and plasma cells, which may obscure the malignant cells (Figure 1). Portions of small intestine away from the tumor mass frequently show typical histopathologic features of celiac disease.18 EATLs typically express cytoplasmic CD3, CD2, CD7, CD103, and cytotoxic markers (TIA-1, perforin, and granzyme-B) and lack CD4, CD5, and CD56 expression. Most cases do not express surface CD3 and TCR. Approximately one-third of patients are CD8+18-20 and intracellular TCRβ expression is detected in one-quarter of patients.21 CD30 expression is common in patients exhibiting anaplastic morphology, but ALK-1 is always negative,17 and the neoplastic T cells are negative for Epstein-Barr virus (EBV).
EATL. (A) Clusters of pleomorphic lymphoma cells reside within a mixed inflammatory background, focally infiltrating the small intestinal epithelium (original magnification, ×500; hematoxylin and eosin [H&E] stain). (B) The large intestine contains a sheet-like proliferation within the lamina propria (original magnification, ×400; H&E stain).
EATL. (A) Clusters of pleomorphic lymphoma cells reside within a mixed inflammatory background, focally infiltrating the small intestinal epithelium (original magnification, ×500; hematoxylin and eosin [H&E] stain). (B) The large intestine contains a sheet-like proliferation within the lamina propria (original magnification, ×400; H&E stain).
MEITL is another primary intestinal lymphoma derived from intraepithelial lymphocytes, which is composed of small- to medium-sized lymphocytes displaying minimal cytomorphologic atypia.4 Although rare in general, MEITL is one of the more frequent subtypes of intestinal lymphoma in Asian and Hispanic populations.20,22 It occurs in older adults (median age 61 years), with a male predominance.21-23 No precursor lymphoproliferative disorder is currently recognized, and in contrast to EATL, there is no definitive association with celiac disease. In MEITL, the neoplastic infiltrate is composed of a monotonous population of small- to medium-sized cells that have oval or round nuclei, fine chromatin, inconspicuous or small nucleoli, and a rim of clear or pale pink cytoplasm (Figure 2). The neoplastic lymphocytes have an overlapping immunophenotype with normal intraepithelial lymphocytes: CD103+, surface and cytoplasmic CD3+, and CD8+. However, the neoplastic lymphocytes commonly express CD56 and invariably express the cytotoxic granule markers, TIA-1 and granzyme-B, but they are negative for CD5.21-24 CD8 can be negative in 12% to 31% of cases and a subset (9%-18%) lack CD56 expression. Although up to 61% of MEITL cases express TCRγ, 16% to 46% of cases are TCRβ+. Up to one-third of cases do not express TCR (TCR silent cases), and up to 16% can be double positive for both TCRγ and β chains.21-23 Aberrant CD20 expression is observed in ∼20% of cases. CD30 is usually negative, and the lymphoma cells lack evidence of EBV infection.21
MEITL. (A) Small- to medium-sized lymphoma cells with dense chromatin and pale cytoplasm display marked epitheliotropism (original magnification, ×1000; H&E stain). (B) CD3 immunohistochemistry highlights a dense mucosal infiltrate of lymphoma cells with striking involvement of the surface epithelium (original magnification, ×400).
MEITL. (A) Small- to medium-sized lymphoma cells with dense chromatin and pale cytoplasm display marked epitheliotropism (original magnification, ×1000; H&E stain). (B) CD3 immunohistochemistry highlights a dense mucosal infiltrate of lymphoma cells with striking involvement of the surface epithelium (original magnification, ×400).
Although the mutational landscapes of MEITL and EATL show extensive overlap, notable differences have been observed. Similar to EATL, the most common mutations in MEITL occur in members of the JAK-STAT (76%-83%) and MAPK (32%-56%) pathways.25-29 Deletions or mutations that inactivate SETD2 are observed in the majority of MEITLs (70%-83%)30 but are comparatively infrequent in EATL,25,31 whereas mutations in other epigenetic modifier genes (CREBBP, EP300, EZH2, ARID1, YLPM1, and TET2) are less frequent in MEITL than EATL. Mutations in GNAI2 appear enriched in MEITL (24% vs 0% in EATL).25,28,32 A recent analysis of 71 MEITL cases confirmed the near universal presence of SETD2 alterations (97%) and defective H3K36 trimethylation, while demonstrating a correlation between TP53 mutations (35%) and MYC expression with atypical morphology. Multivariate analysis found a strong negative impact on outcome in conjunction with MYC expression (>25%), TP53 mutation, STAT5B mutation (57%), and poor performance status, whereas aberrant B-cell marker expression (20%) correlated with better response to treatment and improved OS.33
Presenting signs and symptoms of EATL generally include abdominal pain, diarrhea, nausea, vomiting, weight loss, and B symptoms.17,20,34 Nearly half of patients present with an acute abdomen secondary to intestinal perforation and hemorrhage. Symptoms may be present for weeks to years before the diagnosis of EATL35 or may arise acutely in individuals without known celiac disease. The small intestine is the most frequently affected, although the disease can present in the stomach, colon, and extraintestinal sites (eg, skin and central nervous system).20,36 Typically, the segment of the gastrointestinal tract involved by the lymphoma has an edematous appearance with large circumferential ulcers and occasional plaques.37,38 The lesions can also look similar to celiac disease with loss of mucosal folds. Less commonly EATL can present as bulky, exophytic, or infiltrating masses. Disease can spread to mesenteric lymph nodes, liver, lungs, and bone marrow.17
Approximately half of the patients with MEITL are asymptomatic at diagnosis, whereas, similarly to EATL, the other patients present with an acute abdomen and intestinal perforation. Fewer patients present with prior chronic gastrointestinal symptoms.18,22,23 A solitary mass or multiple, often ulcerated, masses are usually detected on endoscopy, but the small intestinal mucosa can also be diffusely nodular. Tse et al reported that MEITL most often presents in the small intestine alone (63.2%), followed by the large intestine alone (18.4%), and both the small and large intestines (15.8%) as well as small intestine and stomach (5.3%).22 Patients with EATL and MEITL have similarly poor prognosis, with 1-year survival rates of 30% to 40%.3,11,39 Intestinal perforation and infections are common causes of death in both diseases. Severe baseline malnutrition also affects the ability of these patients to even start first-line chemotherapy. Guidelines recommend performing a bone marrow biopsy at staging along with positron emission tomography/computed tomography (PET/CT). A multidisciplinary approach on the best surveillance method should be discussed with gastroenterology and radiology based on disease-specific location and characteristics.
Treatment
There is currently no standard treatment for EATL and MEITL, and in addition to the evolving pathologic classification, most of the data evaluating the effectiveness of treatment are derived from small, retrospective, single-institution reports and/or subgroup analysis of intestinal non-Hodgkin lymphoma studies. Surgery, chemotherapy, and radiotherapy are all used to treat MEITL, although these treatments have shown poor overall outcomes.20,40 Patients treated with anthracycline-based chemotherapy regimens had better survival rates than those treated with surgery and/or radiation therapy.35,41 However, many patients end up not receiving anthracycline-based chemotherapy because they either die before any therapy can be initiated or shortly after surgery for advanced or complicated disease. The Scotland and Newcastle Lymphoma Group retrospectively reported significantly higher progression-free survival (PFS) and overall survival (OS) rates in 54 patients receiving ifosfamide, etoposide, epirubicin/methotrexate (IVE/MTX) followed by autologous stem cell transplant than a historical group of patients receiving anthracycline-based chemotherapy and autologous stem cell transplant.41 This approach was further investigated in a prospective trial that enrolled 11 patients with EATL. Complete remission (CR) was achieved in 55% of patients at the end of the induction treatment with IVE/MTX, with 5 patients (45%) able to proceed to autologous stem cell transplant. For patients with EATL, 1-year OS and PFS were 45%, in contrast with the prior retrospective trial that suggested a 5-year OS and PFS of 60% and 52%, respectively.42 Additional data suggested a role for enteral feeding and gut sterilization before chemotherapy to decrease the risk of perforation and bleeding.43 L-asparaginase has also been used successfully as monotherapy in a few patients not suited for aggressive polychemotherapy, with or without autologous stem cell transplant.44,45
Analyses from a retrospective and a prospective PTCL trial (EATL subgroup) reported an improvement with autologous stem cell transplant in 5-year OS with a rate of 48% to 60% and in 5-year PFS ranging up to 38% to 52%41,46 (Table 1). Updated results from the Nordic NLG-T-01 trial showed a 10-years PFS and OS of both 29%, highlighting the potential for long-term survival with this approach.47 Similar results were found in the largest cohort of patients with EATL, in which the role of autologous stem cell transplant as consolidation or salvage was retrospectively evaluated. In 44 patients with EATL, autologous stem cell transplant yielded a 4-year PFS of 54% and a 4-year OS of 59%.48 Most EATLs are CD30+, and a recent phase 2 trial assessed the role of brentuximab vedotin, cyclophosphamide, doxorubicin, prednisone (Bv-CHP) followed by autologous stem cell transplant for the frontline treatment of patients with EATL. The results showed good tolerance to treatment and high response rates (overall response rate [ORR], 79%; CR, 64%; 2-year PFS, 63%; and 2-year OS, 68%), allowing for the majority of patients to receive transplantation and suggesting that Bv-CHP could be a valuable option for these patients in first line49 (Table 1). MEITL does not generally express CD30, and therefore, this strategy is not an option for these patients, who should instead be treated with an anthracycline-based regimen in first line in the absence of a clinical trial. Age >55 years, poor performance scale, advanced stage, not achieving CR, not receiving autologous stem cell transplant, and presence of TP53 mutations are associated with inferior OS.33,50 In summary, standard chemotherapy, followed by autologous stem cell transplant, remains the preferred option for younger patients with EATL. For very high-risk disease, including MEITL, despite the lack of strong evidence, expert opinion51-53 would also consider allogeneic stem cell transplant in first consolidation. Despite the high transplant-related mortality, this approach remains the preferred option for patients failing autologous stem cell transplant and/or patients with refractory disease54 (Table 1).
In the relapsed and refractory setting, patient enrollment in a clinical trial is strongly preferred but remains challenging. Although novel agents approved for relapsed/refractory PTCL have few or no subjects with these specific subtypes enrolled in their respective registration trials, they continue to be used with variable but overall disappointing results (Table 2). Based on the mutational landscape of these diseases, there is a suggestion that agents such as the JAK1/2 inhibitor ruxolitinib could play a role in treatment of intestinal T-cell lymphomas; however, only 1 patient with MEITL was enrolled into a recently published clinical trial55,56 (Table 2). The aberrant expression of B-cell markers, rare in T-cell lymphoma and recently described in MEITL,33 raises the question of therapies targeting CD20 or CD79. In addition, the loss of H3K36 trimethylation33 may confer high sensitivity to WEE1 kinase inhibitors, which are currently being developed in solid tumors and could be evaluated in MEITL.57 Very interestingly, CD30 chimeric antigen receptor T-cell therapy induced a prolonged remission in a patient with multiply relapsed EATL. At present, this remains anecdotal.58
Overview of important clinical studies in relapsed/refractory PTCL and the representation of rare PTCL subtypes
| Investigational agent used . | Total number of patients . | EATL/MEITL n (best response) . | SPTCL n (best response) . | HSTCL n (best response) . | Responses in other/unspecified subtypes in publication . |
|---|---|---|---|---|---|
| Pralatrexate (Propel study, O’Connor et al)108 | 111 | 0 (0) | 0 (0) | 0 (0) | 3 tMF with response 3 "other" patients with response |
| Romidepsin (Coiffier et al)81 | 130 | 6 (0) | 3 (0) | 0 (0) | NA |
| Belinostat (Belief study, O’Connor et al)83 | 129 | 2 (0) | 0 (0) | 2 (0) | NA |
| Alisertib vs investigator’s choice (Lumiere study, O’Connor et al)109 | 271 | 6 pts total, 3 treated with alisertib, 3 with comparator (NR) | 1, treated with comparator (NR) | 0 (0) | 40 pts with “other” subtypes; ORR in “other” PTCL 32% and 38% for alisertib and comparator respectively |
| Duvelisib – (Pro et al. ASH 2020 and Zinzani et al)85 | 101 | 0 (0) | 1 (CR) | 0 (0) | 4 “other” lymphomas 2 responses seen |
| Ruxolitinib (Moskowitz et al)56 | 53 | 1 (1 patient from the γ/δ TCL group had PR) | 1 (SD) | 2 (1 patient from the γ/δ TCL group had PR) | NA γ/δ TCL group (4 pts) included 1 patient with PCTCL |
| Valemetostat (Horowitz et al.110) | 133 | 1 (NR) | 0 (0) | 0 (0) | 19 patients with “other” subtypes, ORR of 47.4% |
| Golidocitinib (Kim et al.111) | 51 | 2 (0) | 0 (0) | 0 (0) | NA |
| CD70 CAR-T (Iyer et al.112) | 15 | 0 (0) | 0 (0) | 0 (0) | NA |
| CD5 CAR-T (Hill et al.113) | 5 | NR | NR | NR | Responses seen in AITL and PTCL NOS |
| AUTO4 CAR T (Cwynarski et al.114) | 12 | 0 (0) | 0 (0) | 0 (0) | NA |
| AFM13 (Kim et al.115) | 108 | NR | NR | NR | 11 4 patients responded |
| Nivolumab (Bennani et al.116) | 12 | 1 (PR) | 0 (0) | 1 (Hyperprogression) | NA |
| Pembrolizumab (Barta et al.117) | 18 | 1 (Progression) | 0 (0) | 1 (SD) | NA |
| CD30 CAR-T (Vorhees et al.118) | 1 | 1 (CR) | 0 | 0 | NA |
| Investigational agent used . | Total number of patients . | EATL/MEITL n (best response) . | SPTCL n (best response) . | HSTCL n (best response) . | Responses in other/unspecified subtypes in publication . |
|---|---|---|---|---|---|
| Pralatrexate (Propel study, O’Connor et al)108 | 111 | 0 (0) | 0 (0) | 0 (0) | 3 tMF with response 3 "other" patients with response |
| Romidepsin (Coiffier et al)81 | 130 | 6 (0) | 3 (0) | 0 (0) | NA |
| Belinostat (Belief study, O’Connor et al)83 | 129 | 2 (0) | 0 (0) | 2 (0) | NA |
| Alisertib vs investigator’s choice (Lumiere study, O’Connor et al)109 | 271 | 6 pts total, 3 treated with alisertib, 3 with comparator (NR) | 1, treated with comparator (NR) | 0 (0) | 40 pts with “other” subtypes; ORR in “other” PTCL 32% and 38% for alisertib and comparator respectively |
| Duvelisib – (Pro et al. ASH 2020 and Zinzani et al)85 | 101 | 0 (0) | 1 (CR) | 0 (0) | 4 “other” lymphomas 2 responses seen |
| Ruxolitinib (Moskowitz et al)56 | 53 | 1 (1 patient from the γ/δ TCL group had PR) | 1 (SD) | 2 (1 patient from the γ/δ TCL group had PR) | NA γ/δ TCL group (4 pts) included 1 patient with PCTCL |
| Valemetostat (Horowitz et al.110) | 133 | 1 (NR) | 0 (0) | 0 (0) | 19 patients with “other” subtypes, ORR of 47.4% |
| Golidocitinib (Kim et al.111) | 51 | 2 (0) | 0 (0) | 0 (0) | NA |
| CD70 CAR-T (Iyer et al.112) | 15 | 0 (0) | 0 (0) | 0 (0) | NA |
| CD5 CAR-T (Hill et al.113) | 5 | NR | NR | NR | Responses seen in AITL and PTCL NOS |
| AUTO4 CAR T (Cwynarski et al.114) | 12 | 0 (0) | 0 (0) | 0 (0) | NA |
| AFM13 (Kim et al.115) | 108 | NR | NR | NR | 11 4 patients responded |
| Nivolumab (Bennani et al.116) | 12 | 1 (PR) | 0 (0) | 1 (Hyperprogression) | NA |
| Pembrolizumab (Barta et al.117) | 18 | 1 (Progression) | 0 (0) | 1 (SD) | NA |
| CD30 CAR-T (Vorhees et al.118) | 1 | 1 (CR) | 0 | 0 | NA |
AITL, angioimmunoblastic T-cell lymphoma CAR, chimeric antigen receptor; NA, not applicable; PR, partial remission; Pts, patients; SD, stable disease; NR, not reported.
SPTCL
Epidemiology
SPTCL is a rare form of PTCL that was first described in 1991.59-61 Initially, patients with this type of PTCL were reported to have an abysmal outcome, because both αβ and γδ T-cell phenotype lymphomas were included in the SPTCL category.59 In 2005, the WHO-European Organisation for Research and Treatment of Cancer (EORTC) classification called for SPTCL to include only the αβ T-cell phenotype, whereas the γδ cases were included with other types of γδ lymphomas owing to their very aggressive clinical course.60 In clinical practice, αβ vs γδ TCR expression is determined phenotypically by immunohistochemistry or flow cytometry using antibodies that directly target and distinguish between these different receptor subtypes.62 Establishing a diagnosis of SPTCL remains challenging, as evidenced by frequently delayed time to diagnosis, the need for repeated biopsies, and occasional difficulties in differential diagnosis, particularly relating to lupus erythematosus panniculitis.63 A closely related adipotropic lymphoproliferative disorder, in which incomplete histopathological criteria for SPTCL exist, adds to the complexity of obtaining a correct diagnosis in a remarkably rare disease.64 Difficulties distinguishing SPTCL from lupus erythematosus panniculitis still exist, and there is a possibility that both disorders exist on a spectrum.63
A 2001 to 2005 analysis showed that in the United States, SPTCL accounted for only 0.6% of cutaneous lymphomas, with only 23 cases reported.65 A review of Surveillance, Epidemiology, and End Results program data estimated the age-adjusted annual incidence of SPTCL to be <0.1 (per 100 000), with <50 new cases in the United States in 2016 based on modern classification criteria.2 The incidence in Asia is higher, representing anywhere from 2% to 11% of all cutaneous T-cell lymphomas.66,67
Clinical presentation and diagnosis
Morphologically, the malignant lymphocytes in SPTCL create a lobular panniculitis pattern with a characteristic rimming pattern around adipocytes68 (Figure 3). Most lesions feature small- to medium-sized cells, which are positive for CD8, TCR-βF1, and pan–T-cell markers CD2, CD3, CD5, and CD7 (with variable degrees of expression loss), along with cytotoxic markers TIA-1, perforin, and granzyme-B. CD30 and CD56 are negative, as is in situ hybridization for EBV encoded RNA. The proportion of proliferating cells as measured by antibodies to Ki-67 (eg, MIB-1) is high, and there is often a prominent histiocytic component, more pronounced in cases with necrosis.64,68 Although the molecular mechanisms underlying the predisposition for adipose tissue infiltration are not fully understood, the expression of CCR5 on malignant T cells and the secretion of its ligands from immunologically activated adipocytes (CCL3, CCL4, and CCL5) may contribute to the pathogenesis and clinical manifestations of SPTCL.69,70
SPTCL. The lobular subcutaneous infiltrate spares the overlying dermis and epidermis (original magnification ×20; H&E stain). Inset: the lymphoma cells display a characteristic rimming pattern around adipocytes (original magnification ×500; H&E stain).
SPTCL. The lobular subcutaneous infiltrate spares the overlying dermis and epidermis (original magnification ×20; H&E stain). Inset: the lymphoma cells display a characteristic rimming pattern around adipocytes (original magnification ×500; H&E stain).
Review by an expert pathologist is crucial, with potential distinguishing factors including differences in CCR5 expression (significantly higher expression on malignant lymphocytes than lupus erythematosus panniculitis),69 MYC expression (higher in SPTCL over lupus erythematosus panniculitis),71 the presence of so-called Ki-67 hot spots (in SPTCL),72 and paucity of B-cell aggregates and plasmacytoid dendritic cell clusters (in SPTCL).73
Whole-exome sequencing from SPTCL samples revealed that 72% of cases have alterations in epigenetic modifiers, including ARID1B, SMARCA4, CHD4, MBD1, CREBBP, KMT2D, and DOT1L. The same study identified recurrent mutations in PI3K/AKT/mTOR pathway in 44% of cases and included mutations in MTOR, TSC1, PIK3CA, PIK3CD, PIK3CB, TSC2, and AKT2 genes.74 Subsequent work from an international collaboration identified germ line mutations in the HAVCR2 gene, responsible for T-cell immunoglobulin mucin 3 (TIM-3), in ∼60% of SPTCL cases, with mutations following specific geographic distribution.75 TIM-3 is an immune response modulator that acts as a coinhibitory receptor on interferon-γ producing cells, FoxP3+ regulatory T-cell (Treg) cells as well as macrophages and dendritic cells.76 Loss-of-function mutations in TIM-3 lead to its misfolding and perinuclear accumulation, decreased expression on the surface of immune cells, and increased production of inflammatory cytokines, which support the development of hemophagocytic syndrome.75
Subsequent analysis in Korean patients with SPTCL identified HAVCR2 mutation as an adverse prognostic factor for survival. This study also reported on mutations in genes related to immune responses (ASXL1, JAK3, PIAS3, and PLCG2), RNA helicase DDX11, and additional epigenetic modifiers (KMT2C, BAZ2A, and NUP98).77
The median age at presentation of 35 to 40 years is significantly younger for SPTCL than other cutaneous lymphomas, and there is a strong female predominance.64,68 Approximately 20% to 30% of patients with SPTCL have an autoimmune disease, most frequently systemic lupus erythematosus, and up to 15% of patients have a family history of autoimmune disease.64,68,78 It is also noteworthy that disease presents with generalized skin involvement in ∼50% of patients, and extensive lipoatrophy is present in ∼30% of cases.63,64 Hepatosplenomegaly and lymphadenopathy are seen in a minority of patients, whereas constitutional symptoms such as fever, night sweats, and weight loss are present in the majority.64 Approximately 15% to 20% of patients develop hemophagocytic syndrome, which tends to correlate with more extensive disease.64,68
Positron emission tomography is useful in assessing the extent of subcutaneous tissue involvement but is less helpful in assessing nodal stations, because SPTCL tends to spare the lymph nodes and involves only the perinodal fat.64 Additionally, bone marrow biopsy can be considered, especially in the case of localized subcutaneous disease to guide treatment decisions or in cases in which histologic confirmation of hemophagocytosis is needed for hemophagocytic syndrome diagnosis.
Treatment
Due to the rarity of SPTCL, standard therapy is yet to be identified. Overall prognosis is good in cases without hemophagocytic syndrome. Willemze et al reported 5-year OS of 91% in patients without hemophagocytic syndrome compared with a small number of patients with hemophagocytic syndrome in which the 5-year OS was only 49% (most patients were treated with anthracycline-based regimens).68 It is important to note that the course of hemophagocytic syndrome can be quite different in these patients and worsens with disease progression.79,80 A recent retrospective US multicenter study reported that none of the patients (including those with hemophagocytic syndrome) succumbed to their disease.64 Initial treatment modalities included doxorubicin-based therapy (mostly CHOP), which produced CR rate and ORR of 61% and 71%, respectively.68 In patients with no hemophagocytic syndrome or mild hemophagocytic syndrome, less aggressive management with immunomodulatory therapy such as prednisone, cyclosporine, and methotrexate produced CR and ORR of 66.6% and 87.5%, respectively.68 Guitart et al reported that immunomodulatory agents should especially be considered as first line in patients who do not have severe symptoms of disease and/or hemophagocytic syndrome.64 Patients treated with cyclosporine and methotrexate achieved ORR of 94% and 100%, respectively. Additionally, radiation therapy can be used for palliative purposes and in patients with localized disease.64,68 Relapses were common (74% of patients treated with immunomodulatory therapy as front line required additional therapies) but could be managed with other agents. Patients with severe disease/hemophagocytic syndrome might still require aggressive management with chemotherapy and stem cell transplant in first line.63,64 Our recommendation for frontline therapy in SPTCL patients is to use immunomodulatory agents for all asymptomatic or mildly symptomatic patients, including those with mild hemophagocytic syndrome, recognizing the lack of prospective data. Radiotherapy is useful in localized disease and as a palliative option, whereas intensive chemotherapy with stem cell transplant should be tried in frontline setting for patients with symptomatic and aggressive disease.
For relapse/refractory disease, strong evidence is lacking, but a small number of patients with SPTCL have received histone deacetylase inhibitors. Clinically effective use of histone deacetylase inhibitors in SPTCL has been described in case reports, including romidepsin81 and chidamide.82 A pivotal study of romidepsin included 3 patients with SPTCL, however, there were no meaningful responses observed in rare PTCL subtypes. There were no patients with SPTCL in the pivotal phase 2 studies of chidamide82 or belinostat in patients with PTCL83 (Table 2).
Recently, targeted therapies have demonstrated efficacy in SPTCL with examples including the JAK1/2 inhibitor, ruxolitinib,84 and the PI3K inhibitor duvelisib.85 Ruxolitinib in combination with corticosteroids was also effective in 5 pediatric patients with HAVCR2 mutations and panniculitis complicated by hemophagocytic syndrome. Only 2 of these patients fulfilled the histopathologic criteria for SPTCL diagnosis, and 1 of them achieved CR lasting 7 months.86 Our recommendation is that patients with relapse/refractory SPTCL should be offered clinical trial if available.
HSTCL
Epidemiology
HSTCL is a rare and aggressive subtype of PTCL, initially described in 1990 and included as a provisional entity called “γδ hepatosplenic T-cell lymphoma” in the Revised European American Lymphoma Classification (REAL) Classification in 1994.60 After the identification of rare cases with an αβ phenotype, the WHO classification adopted the term “hepatosplenic T-cell lymphoma.”4 Updates in the WHO fifth edition5 and ICC6 did not affect the classification of HSTCL.
Clinical presentation and diagnosis
As its name indicates, the malignant lymphocytes of HSTCL generally display a sinusoidal pattern of infiltration of the liver, spleen, and bone marrow (Figure 4), with most cases featuring small- to medium-sized mature T cells with irregular nuclear contours, mature chromatin, inconspicuous nucleoli, and a moderate amount of agranular cytoplasm. By immunohistochemistry, the malignant cells express surface CD3, CD2, and CD7 and are negative for CD5, CD1a, TdT, and CD10. CD56 is positive in the majority of cases, whereas CD57 is usually negative. The cells generally show a double-negative (CD4−/CD8−) phenotype, although CD8 may be expressed in some cases. Cytotoxic granule–associated markers TIA-1 and granzyme-M are typically positive, whereas perforin and granzyme-B are negative, in keeping with a nonactivated cytotoxic phenotype. There is no association with EBV.87 The neoplastic T cells of HSTCL typically arise from a subset of γδ TCR–expressing lymphocytes; however, the αβ disease variant occurs more commonly in women and in patients aged >50 years and has been associated with worse prognosis.88
HSTCL. (A) The hepatic sinusoids are filled and expanded by lymphoma cells (original magnification ×1000; H&E stain). (B) The splenic red pulp is extensively infiltrated (original magnification, ×500; H&E stain).
HSTCL. (A) The hepatic sinusoids are filled and expanded by lymphoma cells (original magnification ×1000; H&E stain). (B) The splenic red pulp is extensively infiltrated (original magnification, ×500; H&E stain).
The most common chromosomal abnormalities in HSTCL are isochromosome 7q [i(7q)] observed in ∼25% to 70% of cases and trisomy 8 observed in 8% to 53% of cases. Less common alterations include 7q amplification, loss of Y chromosome, loss of chromosome 10q (19%), and gains in chromosome 1q (13%).89 A recent study also showed gain/amplifications of 7q22.1, 1q31.1, and overexpression of genes at this locus including ABCB1, RUNDC3B, and PPP1R9A. Gene expression profiling showed overexpression of genes encoding natural killer–related antigens including killer immunoglobulin-like receptors, as well as upregulation of oncogenes (MYBL1 and VAV3), cell-trafficking genes (S1PR5), a multidrug resistance 1 (MDR-1) gene, and downregulated tumor-suppressor genes such as AIM1.90 Whole-exome sequencing analysis of 68 HSTCL cases revealed that the most frequently mutated genes included chromatin-modifying genes, affecting 62% of cases. Common epigenetic alterations included mutations in SETD2 (25%), INO80 (21%), TET3 (15%), and SMARCA2 (10%).91 This analysis also confirmed the presence of recurrent somatic mutations of PIK3CD and missense mutations of STAT5B and STAT3 genes in HSTCL.29,92 Other recurrent driver mutations were observed in TP53, UBR5, and IDH2.
Clinical presentation, histologic features, and molecular findings make this disease a unique entity among other PTCLs. Patients with HSTCL frequently report systemic symptoms, including fatigue, night sweats, weight loss, and fever. HSTCL occurs predominantly in young adults, with a median age of 34 years and strong male predominance.87 Cytopenias are very common, whereas lymphadenopathy is typically absent. Although lymphocytosis is uncommon, a small population of atypical lymphocytes can be detected by flow cytometry in ∼50% of patients. Other laboratory findings include elevated lactate dehydrogenase and abnormal liver function tests. Hemophagocytic syndrome can also occur and is generally associated with a rapid and aggressive disease course. Given its rarity and the absence of nodal involvement in most cases, diagnosis of HSTCL can be challenging, and the disease can mimic infectious etiologies or other malignant disorders including T-large granular lymphocytic leukemia, natural killer–cell leukemia, and chronic active EBV disease. Staging workup for HSTCL is the same as that routinely used for other non-Hodgkin lymphoma, with the exception of bone marrow aspiration and biopsy, which are essential for baseline evaluation in HSTCL. Most cases of HSTCL occur de novo, but ∼20% of cases arise in immunocompromised patients, with reports of HSTCL developing during long-term immunosuppression after solid-organ transplant and in the setting of other immune dysregulation including malignancy and infection. The importance of iatrogenic immunosuppression as a contributor to lymphomagenesis has become particularly relevant in light of the increased incidence of HSTCL in patients with chronic inflammatory diseases after treatment with immunosuppressants, specifically agents blocking tumor necrosis factor α and/or thiopurine agents.93 Risk factors for HSTCL in inflammatory bowel disease are young age, concomitant use of anti–tumor necrosis factors, Crohn disease as an inflammatory bowel disease subtype, male sex, and long-term thiopurine therapy.94
Treatment
Due to the rarity of the disease, with only a few hundred cases described in the literature, there is no standard of care for patients with HSTCL. The prognosis of HSTCL is very poor, and no prospective trials investigating treatment approaches have been reported.95
Outcomes with standard anthracycline-containing induction regimens have been disappointing with variable responses, high relapse rates, and short median survival times.96-98 In a limited number of patients, a more intensive regimen, fractionated cyclophosphamide, liposomal doxorubicin, vincristine, and dexamethasone (HyperCVIDDoxil),96 alternating with methotrexate and high-dose cytarabine, resulted in a higher rate of CR. In a retrospective, single-center institution analysis, 14 patients with HSTCL received alternative, nonanthracycline, induction chemotherapy regimens. Seven of 14 remained alive, with a median follow-up of 66 months. Six of the 7 received induction chemotherapy regimens such as ICE (ifosfamide, carboplatin, and etoposide) or IVAC (ifosfamide, etoposide, and high-dose cytarabine) as opposed to CHOP, and all surviving patients proceeded to undergo either autologous stem cell transplant or allogeneic stem cell transplant. All patients who received a stem cell transplant were alive and in CR at the time of the analysis, suggesting that this modality can be potentially curative99 (Table 1). Several authors have published experiences with autologous stem cell transplant or allogeneic stem cell transplant, and a 2007 collection of published case reports of HSTCL treated with allogeneic stem cell transplant suggests better outcomes with this approach.100 Splenectomy can be a useful therapeutic option in selected patients, particularly in patients with significant thrombocytopenia, which can limit the administration of full doses of chemotherapy.101 Overexpression of MDR-1 and pgp-1 amplification observed in HSTCL and other T-cell lymphoma subtypes can explain the intrinsic refractory nature of the disease and support the use of non-MDR–susceptible agents.90 Expert opinion is that based on the disappointing data with anthracycline-based chemotherapy, patients with HSTCL should be treated with ifosfamide–based induction treatment and consolidated with allogeneic stem cell transplant in first remission,51-53,98 because long-term remission is primarily or exclusively seen in those who have undergone consolidative allogeneic stem cell transplant in first remission.102
In the setting of relapsed/refractory HSTCL, the strongest recommendation is for enrollment into clinical trial. Anecdotal reports of activity of other chemotherapy agents including alemtuzumab and purine analogs (pentostatin, fludarabine, and cladribine) in single patients has been described in case reports and should be considered.103-105
Conclusion
In summary, although many of the key clinicopathologic features of these diseases have been described and validated, their rarity has undoubtedly contributed to a slower pace of research and limitations on the amount of high-quality, evidence-based data that physicians can use to guide therapeutic decisions. The presence of recurrently mutated pathways (eg, JAK-STAT), widespread epigenetic dysregulation, and the intrinsic chemo-refractoriness of these rare PTCLs suggest that critical aspects of their biology and drug resistance could potentially be overcome by the development of innovative therapeutic strategies.56 However, our own analysis of the most recently published trials of novel single agents and drug combinations in relapse/refractory PTCL reveals that patients with these rare entities are represented in single digits if not completely absent and once again demonstrates the lack of standard of care in these diseases and the excruciating need for dedicated focus from the scientific community (Table 2).
Acknowledgment
E.M. is funded under Office of Orphan Products Development grant RO1 no. FD-R-006814-01.
Authorship
Contribution: E.M. collected the data and wrote the manuscript; and J.W.C. and M.K. collaborated on the manuscript preparation.
Conflict-of-interest disclosure: E.M. reports research funding from Merck, Celgene/Bristol Myers Squibb, Astex Pharmaceuticals, Kymera Therapeutics, and Dren Bio; scientific advisory roles for Vittoria Biotherapeutics, Kyowa Kirin Seagen, and Dren Bio; and being a member of Everest Clinical Research Data Safety Monitoring Committee. J.W.C. reports consultancy fees from Bayer; and honorarium from BeiGene. M.K. is a stockholder at Pfizer Inc.
The current affiliation for M.K. is Pfizer Inc, New York, NY.
Correspondence: Enrica Marchi, Division Hematology – Oncology, Department of Medicine, University of Virginia Cancer Center, 1300 Jefferson Park Ave, West Complex, rm 6002, Charlottesville, VA 22908-0716; email: em5yt@uvahealth.org.
References
Author notes
The online version of this article contains a data supplement.

![EATL. (A) Clusters of pleomorphic lymphoma cells reside within a mixed inflammatory background, focally infiltrating the small intestinal epithelium (original magnification, ×500; hematoxylin and eosin [H&E] stain). (B) The large intestine contains a sheet-like proliferation within the lamina propria (original magnification, ×400; H&E stain).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/18/10.1182_blood.2023021788/2/m_blood_bld-2023-021788-c-gr1.jpeg?Expires=1769899122&Signature=sW-FwNHCCpJXpr23csVQ9qYgnIoB9zfbm3lHqThlEyvDTft2MwOlN3~0u7vVN5rIeTZhfLGvJhT8HxpxbmvQIbLJJUBu50ccyjelsaNXz45ZQZ3tg8m3m0V~agGxEbUlHL20RBCrJe0~diGc9wuMpHLGwDQWyciIAtUJsc1Fl-WFoA4qs6GoNp6Im5VRZTlaVdUhKMU9YOFISWEvWItcQVuuipXl3~nHsvhiifNybIE9Yf~2hj6zZr8-IK8IIo-iX58RilR-x7kVaunDjDbjGbbIOCRjLJEA9HGJYnGlwarLKZbTEfBBp7Z4Sh5tEavHhNZhSkrWaOvEZ0eidULKlw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal