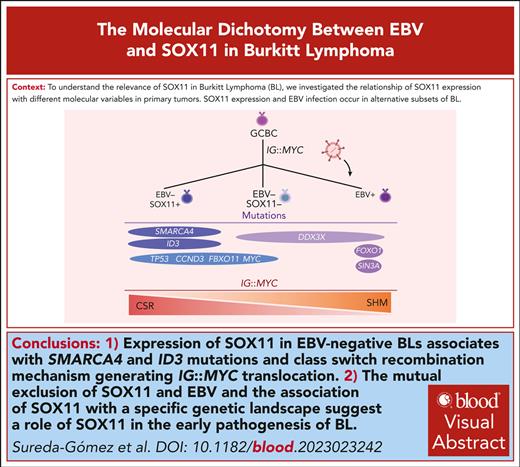
Key Points
SOX11 expression and EBV infection occur in alternative subsets of BL with different profiles of somatic mutations.
Among EBV− BL, IG∷MYC translocation is generated by class switch recombination in SOX11+ BL and somatic hypermutation in SOX11− tumors.
Visual Abstract
SRY-related HMG-box gene 11 (SOX11) is a transcription factor overexpressed in mantle cell lymphoma (MCL), a subset of Burkitt lymphomas (BL) and precursor lymphoid cell neoplasms but is absent in normal B cells and other B-cell lymphomas. SOX11 has an oncogenic role in MCL but its contribution to BL pathogenesis remains uncertain. Here, we observed that the presence of Epstein-Barr virus (EBV) and SOX11 expression were mutually exclusive in BL. SOX11 expression in EBV-negative (EVB-) BL was associated with an IG∷MYC translocation generated by aberrant class switch recombination, whereas in EBV-negative (EBV−)/SOX11-negative (SOX11−) tumors the IG∷MYC translocation was mediated by mistaken somatic hypermutations. Interestingly, EBV− SOX11-expressing BL showed higher frequency of SMARCA4 and ID3 mutations than EBV−/SOX11− cases. By RNA sequencing, we identified a SOX11–associated gene expression profile, with functional annotations showing partial overlap with the SOX11 transcriptional program of MCL. Contrary to MCL, no differences on cell migration or B-cell receptor signaling were found between SOX11− and SOX11-positive (SOX11+) BL cells. However, SOX11+ BL showed higher adhesion to vascular cell adhesion molecule 1 (VCAM-1) than SOX11− BL cell lines. Here, we demonstrate that EBV− BL comprises 2 subsets of cases based on SOX11 expression. The mutual exclusion of SOX11 and EBV, and the association of SOX11 with a specific genetic landscape suggest a role of SOX11 in the early pathogenesis of BL.
Introduction
Burkitt lymphoma (BL) is a highly proliferative B-cell neoplasm that originates from germinal center B cells.1 It is the most common B-cell lymphoma in children and adolescents but also occurs in adults.2,3 Three clinical variants are distinguished: endemic BL (eBL), sporadic BL (sBL), and immunodeficiency-related BL. eBL is usually positive for Epstein-Barr virus (EBV), occurs mainly in countries of central Africa in which malaria is endemic, and presents with jaw or facial bone involvement in pediatric patients. Clinically, sBL differs from eBL because it involves mostly the abdomen (Peyer patches); head and neck lymph nodes; and, in some cases, the bone marrow.1,4,5 Moreover, sBL is less commonly positive for EBV. However, when detected in sBL, EBV is more frequent in adult cases.6,7
The genetic hallmark of BL is the MYC rearrangement to 1 of the immunoglobulins (IG) loci, leading to the constitutive overexpression of MYC.8-10MYC dysregulation in B cells is not sufficient for BL development and additional genomic changes are required.11,12 Interestingly, several studies have revealed important genetic and molecular differences depending on the EBV status of patients with BL.13-21
BL is 1 of the few lymphomas that shows expression of the SRY-related HMG-box gene 11 (SOX11).22-24 SOX11 expression in BL occurs in 25% to 55% of tumors, predominantly in pediatric patients.7,24,25 Moreover, SOX11 expression is included in the transcriptional molecular signature used to classify BL.26 SOX11 expression in MCL is characteristic of the conventional molecular subtype with worse outcome than the SOX11-negative (SOX11−) leukemic nonnodal MCL subtype.27 In contrast, no association between SOX11 expression and survival has been found in BL.24 Several in vitro and in vivo studies have shown the oncogenic role of SOX11 in the pathogenesis of MCL.28-34 However, the contribution of SOX11 expression to BL pathogenesis remains unknown.
To understand the relevance of SOX11 in BL, we have investigated the relationship of SOX11 expression with different molecular variables in primary tumors and evaluated the modulation of gene expression profiles (GEP) and functional changes upon SOX11 overexpression and knockout (KO) in BL cell lines.
Methods
Cell lines
SOX11− BL cell lines Ramos and DG75 (American Type Culture Collection [ATCC] Cancer Research Line [CRL]-1596 and ATCC CRL-2625, respectively); and the SOX11-positive (SOX11+) BL cell line BL2 (DSMZ ACC 625) were used to generate stable transduced DG75 ER-SOX11+ and Ramos SOX11+, ectopically overexpressing SOX11, and BL2-SOX11 KO BL cell lines. Moreover, we used the stable transduced Z138-SOX11KO34 and JVM2 (JVM2-SOX11+)32,34 and its control (Z138CT and JVM2CT) MCL cell lines, previously generated by our laboratory. See more details on cell culture, plasmids, and generation of stable transduced BL cell lines in supplemental Methods, available on the Blood website.
BL cohorts
Four previously published BL series were used to correlate SOX11 expression or positivity with different BL molecular characteristics. Duplicated cases between series have been considered and ruled out from 1 of the duplicated series. The BL Genome Sequencing Project (BLGSP)21 includes 117 pediatric patients with BL (96 with eBL, and 21 with sBL) with available RNA sequencing (RNA-seq) and molecular data, including EBV status, breakpoint of IG::MYC, and recurrently mutated genes, obtained by whole-genome sequencing (WGS). The Richter et al cohort7 includes 138 patients with sBL (80 children and 58 adults) with available SOX11 immunohistochemistry (IHC), EBV–encoded small nuclear RNA (EBER) in situ hybridization (ISH) and targeted DNA-sequencing data (79 cases). The Burkhardt et al cohort35 includes sBL cases with deep targeted DNA-sequencing data. International Cancer Genome Consortium Molecular Mechanisms of Malignant Lymphoma with Sequencing (ICGC MML-Seq) cases16 include 24 pediatric sBL cases with available SOX11 IHC and molecular data, including EBV status, breakpoint of IG::MYC, and recurrently mutated genes, obtained by WGS. All pediatric patients were aged <20 years, and the adults ≥20 years.
SOX11 status
IHC of SOX11 was performed for 51 pediatric BL cases with available formalin-fixed paraffin-embedded (FFPE) tissue35 on an automated strainer (Leica) by using a mouse monoclonal antibody against SOX11 (MRQ-58; Cell Marque) and a pH-6 antigen retrieval solution, as previously described.36,37 SOX11 IHC was previously obtained for the ICGC MMML-Seq16 and Richter et al cohorts.7 SOX11 was scored positive when at least 10% of lymphoma cells showed unambiguous nuclear staining although in most cases the majority of neoplastic cells rather than small subsets were positive for SOX11. In BLGSP cases for which no IHC was possible,21 SOX11+ expression was defined as >10.5 log2–transformed value, obtained by RNA-seq data.
EBV studies
We performed EBER ISH in 51 pediatric BL cases with available FFPE biopsy specimens35 using Leica BOND-MAX staining systems and reagents (Leica). EBV positivity was defined as most tumor cells being positive. Presence of EBV traces were investigated by droplet digital polymerase chain reaction (ddPCR) for Epstein-Barr virus nuclear antigen 1 (EBNA1) and BamHI in a cohort of 37 BL specimens negative for EBER (n = 12 SOX11+, and n = 25 SOX11−), as previously described (supplemental Methods).38,39 Combined IHC for CD10 and the RNAscope assay for EBNA1 was performed using Leica BOND III automated system (Leica, Wetzlar, Germany) in FFPE BL samples EBER-ISH negative but positive by ddPCR (supplemental Methods).38,39
Molecular profiling
The IG region involved and the mechanism leading to the IG::MYC translocation were evaluated in 89 BL cases from the BLGSP21 and 24 BL cases from ICGC MMML-Seq cohort.16IG::MYC translocations with breakpoints on the IG loci localized inside or near (<500 base pairs) of class switch regions of IGH were classified as translocations mediated by class switch recombination (CSR), while those inside variable diversity joining (V[D]J) regions but far from recombination signal sites (>15 base pairs) were classified as mediated by somatic hypermutation (SHM). Translocations with breakpoints localized close to recombination signal sites (<15 base pairs) were classified as mediated by recombination activating gene (RAG) during V(D)J recombination.
For mutational analysis in patients with BL, lists of mutated driver genes were obtained from the different publications for a total of 267 patients after excluding overlapped cases.7,16,21,35 Some of the data were obtained by WGS and the other by targeted mutational analysis. For targeted mutational analysis, only mutations with a variant allele frequency of ≥10% were considered. Only genes that were mutated in ≥10% of the BL cases were used for OncoPrint analysis and comparisons between groups.
RNA-seq
RNA was obtained using the RNeasy Mini RNA extraction kit (Qiagen) following manufacturer instructions. RNA quality was checked using a Bioanalyzer (Agilent) and messenger RNA (mRNA) libraries were prepared using the TruSeq stranded mRNA kit for DG75 cell lines, or the TruSeq RNA Library Prep Kit version 2 for Ramos and BL2 cell lines (Illumina). Samples were sequenced on NovaSeq or NextSeq2000 sequencers. For each condition, 3 technical replicates were analyzed.
RNA-seq data from DG75 cell lines were analyzed using the open source web-based Galaxy.40 Paired-end FASTQ files were aligned to the human genome (GRCh38) using HISAT2. Counts files were generated with featureCounts using GRCh38.102.gtf as gene annotation file. For Ramos and BL2 cell lines, single-end sequencing reads were processed and aligned, as previously described. Gene count matrix was obtained for BLGSP21 BL primary cases. RNA-seq data analyses are detailed in supplemental Methods.
Statistics
Methods are described in the supplemental Methods.
The study was approved by the ethics committee of the Medical Faculty of the University of Kiel (D 429/13) and conducted in accordance with the Declaration of Helsinki.
Results
SOX11 expression and EBV infection are mutually exclusive in BL
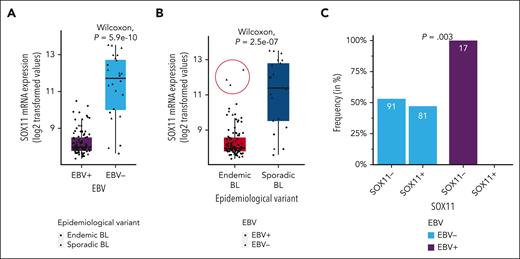
We first investigated the association between SOX11 expression and different molecular characteristics of the tumors and clinical features of the patients. Using previously published RNA-seq and clinical data from 117 pediatric BLs,21 we observed significantly higher SOX11 mRNA levels in EBV− than EBV+ tumors (Figure 1A and B, respectively). To confirm this observation, we used 189 cases of 2 independent series of pediatric and adult patients with sBL,7,35 performing SOX11 IHC and EBER ISH in the FFPE tissue sections of tumors in which these data were not available (supplemental Table 1 and supplemental Figure 1A-F). None of the 17 EBV+ BL (0% with 95% confidence interval, 0-19.5), whereas 81 of 172 EBV− BLs were positive for SOX11 (47.1%, with 95% confidence interval, 39.5-54.8), showing mutual exclusivity between SOX11 positivity and EBV positivity (P = .003; Figure 1C). SOX11 expression was significantly associated with sBL but not exclusively because 3 BL cases from endemic areas showed high SOX11 mRNA expression, all of them EBV−, comparable with those observed in SOX11+ sBL cases (Figure 1B, red circle). No other molecular or clinical features differed between these 3 cases compared with the rest of the eBL cases (all 3 were HIV− [R. Morin, written communication, 8 Jan 2024]). Unfortunately, SOX11 IHC was not available for these cases.21
SOX11 is exclusively expressed in EBV− BL cases. (A-B) SOX11 mRNA expression (log2-transformed values) according to EBV status (EBV− and EBV+) (A) and epidemiological variant (endemic BL and sporadic BL) (B) of 117 pediatric BL cases. Red circle highlights high SOX11 expression in 3 BLs from endemic areas. Wilcoxon text was performed to test differences between groups. (C) Frequency of patients who are SOX11+ and SOX11− (by IHC) in an independent series of pediatric and adult sBLs (n = 189), according to EBV status. The Cochran-Mantel-Haenszel test was performed to test differences between the frequency in EBV− and in EBV+, while adjusting for cohort and group.
SOX11 is exclusively expressed in EBV− BL cases. (A-B) SOX11 mRNA expression (log2-transformed values) according to EBV status (EBV− and EBV+) (A) and epidemiological variant (endemic BL and sporadic BL) (B) of 117 pediatric BL cases. Red circle highlights high SOX11 expression in 3 BLs from endemic areas. Wilcoxon text was performed to test differences between groups. (C) Frequency of patients who are SOX11+ and SOX11− (by IHC) in an independent series of pediatric and adult sBLs (n = 189), according to EBV status. The Cochran-Mantel-Haenszel test was performed to test differences between the frequency in EBV− and in EBV+, while adjusting for cohort and group.
Together, these results suggest that SOX11 expression delineates a different molecular subtype of EBV− BL. To analyze this hypothesis, we established 3 different groups of patients according to their EBV and SOX11 status: EBV+ (all SOX11−), EBV−/SOX11− “double negative,” and EBV−/SOX11+, and analyzed its association with different molecular BL features.
IG::MYC translocation is predominantly generated by CSR in EBV−/SOX11+ BL and by SHM in EBV−/SOX11− BL
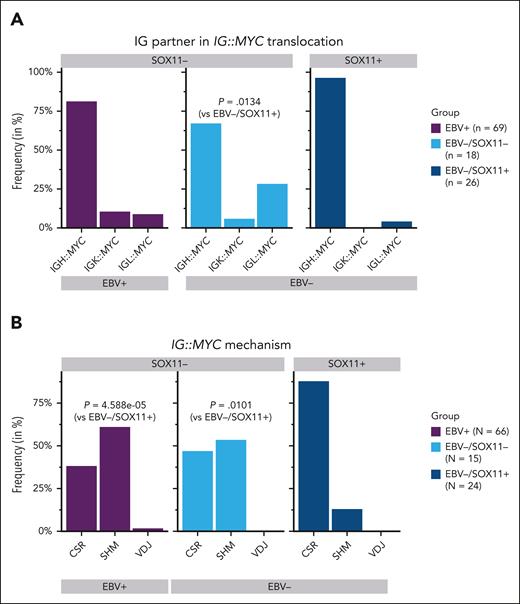
To study the IG partners and the mechanisms involved in the generation of IG::MYC translocation according to EBV and SOX11 status in BL cases, we used the breakpoints of the IG::MYC translocation in 24 and 89 pediatric patients with BL from the ICGC MMML-Seq16 cohort and the BLGSP,21 respectively, for which these data were available. The SOX11 status was determined by IHC in ICGC MMML-Seq BL cases, and by RNA-seq data in the BLGSP BL cases (supplemental Figure 1G). We observed that 56 of 69 (81%) EBV+, 25 of 26 (96%) EBV−/SOX11+, and 12 of 18 (67%) EBV−/SOX11− double negative BL carried an IGH::MYC translocation, and in lower proportion, IGL::MYC or IGK::MYC translocations with IG partner frequencies being significantly different in EBV−/SOX11− double negative and EBV−/SOX11+ cases (P = .0134; Figure 2A; supplemental Table 2).
IG::MYC translocation in BL primary cases according to SOX11 expression levels and EBV status. (A-B) Frequency of IGH::MYC, IGK::MYC, and IGL::MYC translocations (A), and frequency of translocations generated by CSR, SHM, or V(D)J processes (B), in the total group of EBV+, EBV−/SOX11−, and EBV−/SOX11+ BLs. Fisher exact test was performed to evaluate differences between group frequencies.
IG::MYC translocation in BL primary cases according to SOX11 expression levels and EBV status. (A-B) Frequency of IGH::MYC, IGK::MYC, and IGL::MYC translocations (A), and frequency of translocations generated by CSR, SHM, or V(D)J processes (B), in the total group of EBV+, EBV−/SOX11−, and EBV−/SOX11+ BLs. Fisher exact test was performed to evaluate differences between group frequencies.
The potential mechanism mediating the translocation, considering the 3 IG loci, was determined in 105 of the total 113 BL cases for which these data were available, finding significant differences between the 3 groups (P = .0002). We found the breakpoint in a class switch region of the IGH, in 87% of the EBV−/SOX11+ (21/24) but only in 47% of the EBV−/SOX11− double-negative (7/15) BL and 38% of the EBV+ BL (25/66). At the same time, 12.5% of the EBV−/SOX11+ (3/24), 53% of the EBV−/SOX11− double negative (8/15), and 61% of the EBV+ (40/66) BL cases had the breakpoint located in the V(D)J region as a result of SHM process. Only 1 case showed evidence of acquisition of the translocation by aberrant V(D)J recombination mediated by RAG in the group of EBV+ BLs (Figure 2B; supplemental Table 2). Pairwise comparisons showed significant differences in the mechanism mediating the translocation between EBV−/SOX11+ and EBV−/SOX11− double negative (P = .005), and between EBV−/SOX11+ and EBV+ BLs (P = 4.6 × 10−5).
As previously described,21 significantly lower AICDA mRNA expression levels were observed in EBV−/SOX11+ (P = 2.4 × 10−9) and EBV−/SOX11− (P = 0.052) compared to EBV+ BLs. However, no significant differences were observed between EBV−/SOX11− and EBV−/SOX11+ BLs (P = 0.21; supplemental Figure 2).
Together, these data suggest that among EBV− BL, SOX11 status is associated with the early pathogenetic event of the MYC translocation.
SOX11+ cases have a distinct mutational landscape among EBV− BL
Several genes are recurrently mutated in BL, promoting oncogenic mechanisms responsible for the development of tumor cells.14 We combined previously published data on recurrently mutated genes obtained by WGS from 117 pediatric patients with eBL and sBL from the BLGSP,21 and from 24 pediatric sBL cases from the ICGC MMML-Seq cohort,16 and targeted mutational data on driver BL genes of 2 different series with 79 pediatric and adults,7 and 47 pediatric35 sBL cases, receptively. We identified 17 coding genes mutated in ≥10% of BL cases (Figure 3A; supplemental Table 3).
Mutational profile of BL primary cases according to EBV status and SOX11 expression. (A) Mutational analysis in recurrently mutated driver genes (mutated in ≥10% of cases) of 267 BLs. Mutations, EBV and SOX11 status, age group (pediatric or adult), and epidemiological variant (endemic or sporadic) are shown. The cases in the heat map are ordered by SOX11 and EBV status. (B) Frequencies of mutated cases in EBV+ (n = 110), EBV−/SOX11− (n = 76), and EBV−/SOX11+ (n = 81) for each gene are shown. Fisher exact test with false discovery rate correction was performed to evaluate differences in the frequencies between groups. ∗∗∗∗q value < 0.0001; ∗∗∗q value < 0.001; ∗∗q value < 0.01; ∗q value < 0.15.
Mutational profile of BL primary cases according to EBV status and SOX11 expression. (A) Mutational analysis in recurrently mutated driver genes (mutated in ≥10% of cases) of 267 BLs. Mutations, EBV and SOX11 status, age group (pediatric or adult), and epidemiological variant (endemic or sporadic) are shown. The cases in the heat map are ordered by SOX11 and EBV status. (B) Frequencies of mutated cases in EBV+ (n = 110), EBV−/SOX11− (n = 76), and EBV−/SOX11+ (n = 81) for each gene are shown. Fisher exact test with false discovery rate correction was performed to evaluate differences in the frequencies between groups. ∗∗∗∗q value < 0.0001; ∗∗∗q value < 0.001; ∗∗q value < 0.01; ∗q value < 0.15.
Then, we analyzed the frequency of mutations of these 17 genes in the 3 groups of patients with BL previously established: EBV+ (all SOX11−), EBV−/SOX11−, and EBV−/SOX11+. We observed significant differences in the frequency of CCND3, DDX3X, FBXO11, FOXO1, ID3, MYC, SIN3A, SMARCA4, and TP53 mutations between these groups of patients (q value < 0.1; Figure 3B; supplemental Table 4). Pairwise comparisons showed that EBV−/SOX11− double negative and EBV−/SOX11+ BLs share a higher frequency of mutations in CCND3, ID3, and TP53 genes, and lower in FOXO1 gene relative to EBV+ BLs (q value < 0.1). However, EBV+ cases showed a significant higher frequency of mutations in DDX3X and SIN3A, and fewer in MYC than EBV−/SOX11+, but not to EBV−/SOX11− double negative BL (q value < 0.05), suggesting that these differences cannot be attributed to the EBV status alone. In addition, among EBV− cases, EBV−/SOX11+ BL had a significantly higher frequency of mutations in SMARCA4 and ID3 (43% and 80%, respectively) than EBV−/SOX11− double negative (18% and 63%, respectively; q value = 0.14 in both comparisons). As expected, SMARCA4 and ID3 were also less frequently mutated in EBV+ compared with EBV−/SOX11+ cases (9% and 35%, respectively; q-values < 0.001; Figure 3B and supplemental Table 5). Thus, both EBV infection7,20,21 and SOX11 expression in BL are associated with a distinct mutational pattern.
Highly sensitive detection of EBV in SOX11/EBER double negative BL
Because SOX11 expression and EBV detected by the gold standard method EBER ISH leaves a third group of BL, being negative for both features (double negative), more common in adult patients (Figure 3A), we asked whether EBER ISH may miss the detection of EBV in these BLs. Thus, a cohort of 37 BL samples that were negative for EBER (n = 14 SOX11+ and n = 23 SOX11−) were blindly tested for traces of EBV by ddPCR for both EBNA1 and BamHI-W conserved regions of the EBV genome, as previously described.38 Twelve cases (32.4%) were positive for BamHI-W (0.19-18 copies per μL) of which 9 were also positive for EBNA1 (0.19-2.7 copies per μL). In 6 cases, the presence of EBV sequences in tumor cells were confirmed by dual staining with RNAscope for EBNA1 and IHC for CD10, showing colocalization of EBV-specific signals in CD10+ lymphoma cells. The vast majority of BLs with traces of EBV were SOX11− (10/12; 83.3%), whereas only 2 (2/12; 16.7%) were SOX11+ (Table 1).
Detection of EBV genome traces obtained by ddPCR of BamHI-W and EBNA1, and RNAscope of EBNA1, in a cohort of 37 BL specimens
| Study ID . | EBER ISH . | SOX11 IHC . | BamHI-W (copies per μL) . | EBNA1 (copies per μL) . | EBNA1 mRNA (score) . |
|---|---|---|---|---|---|
| BL-42 | EBV− | SOX11− | 1.01 | 0 | NA |
| BL-53 | EBV− | SOX11− | 6.3 | 1.7 | 9 |
| BL-48 | EBV− | SOX11− | 0 | 0 | NA |
| BL-54 | EBV− | SOX11− | 0.19 | 0 | NA |
| BL-3 | EBV− | SOX11− | 15 | 2.6 | 9 |
| BL-34 | EBV− | SOX11− | 0 | 0 | NA |
| BL-17 | EBV− | SOX11− | 15 | 1.42 | 7 |
| BL-1 | EBV− | SOX11− | 0 | 0 | NA |
| BL-29 | EBV− | SOX11− | 18 | 1.25 | 6 |
| BL-10 | EBV− | SOX11− | 0.2 | 0.2 | NA |
| BL-7 | EBV− | SOX11− | 0 | 0 | NA |
| BL-31 | EBV- | SOX11− | 0 | 0 | NA |
| BL-56 | EBV− | SOX11− | 0 | 0 | NA |
| BL-51 | EBV− | SOX11− | 0.31 | 0 | NA |
| BL-46 | EBV− | SOX11− | 0 | 0 | NA |
| BL-43 | EBV− | SOX11− | 0.62 | 0.25 | 6 |
| BL-15 | EBV− | SOX11− | 0 | 0 | NA |
| BL-89 | EBV− | SOX11− | 0 | 0 | NA |
| BL-2 | EBV− | SOX11− | 0 | 0 | NA |
| BL-18 | EBV− | SOX11− | 0 | 0 | NA |
| BL-113 | EBV− | SOX11− | 1.31 | 0.5 | NA |
| BL-119 | EBV− | SOX11− | 0 | 0 | NA |
| BL-38 | EBV− | SOX11− | 0 | 0 | NA |
| BL-143 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-144 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-41 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-105 | EBV− | SOX11+ | 0.66 | 0.19 | NA |
| BL-25 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-12 | EBV− | SOX11+ | 15.9 | 2.7 | 11 |
| BL-32 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-50 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-116 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-72 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-145 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-146 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-147 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-148 | EBV− | SOX11+ | 0 | 0 | NA |
| Study ID . | EBER ISH . | SOX11 IHC . | BamHI-W (copies per μL) . | EBNA1 (copies per μL) . | EBNA1 mRNA (score) . |
|---|---|---|---|---|---|
| BL-42 | EBV− | SOX11− | 1.01 | 0 | NA |
| BL-53 | EBV− | SOX11− | 6.3 | 1.7 | 9 |
| BL-48 | EBV− | SOX11− | 0 | 0 | NA |
| BL-54 | EBV− | SOX11− | 0.19 | 0 | NA |
| BL-3 | EBV− | SOX11− | 15 | 2.6 | 9 |
| BL-34 | EBV− | SOX11− | 0 | 0 | NA |
| BL-17 | EBV− | SOX11− | 15 | 1.42 | 7 |
| BL-1 | EBV− | SOX11− | 0 | 0 | NA |
| BL-29 | EBV− | SOX11− | 18 | 1.25 | 6 |
| BL-10 | EBV− | SOX11− | 0.2 | 0.2 | NA |
| BL-7 | EBV− | SOX11− | 0 | 0 | NA |
| BL-31 | EBV- | SOX11− | 0 | 0 | NA |
| BL-56 | EBV− | SOX11− | 0 | 0 | NA |
| BL-51 | EBV− | SOX11− | 0.31 | 0 | NA |
| BL-46 | EBV− | SOX11− | 0 | 0 | NA |
| BL-43 | EBV− | SOX11− | 0.62 | 0.25 | 6 |
| BL-15 | EBV− | SOX11− | 0 | 0 | NA |
| BL-89 | EBV− | SOX11− | 0 | 0 | NA |
| BL-2 | EBV− | SOX11− | 0 | 0 | NA |
| BL-18 | EBV− | SOX11− | 0 | 0 | NA |
| BL-113 | EBV− | SOX11− | 1.31 | 0.5 | NA |
| BL-119 | EBV− | SOX11− | 0 | 0 | NA |
| BL-38 | EBV− | SOX11− | 0 | 0 | NA |
| BL-143 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-144 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-41 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-105 | EBV− | SOX11+ | 0.66 | 0.19 | NA |
| BL-25 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-12 | EBV− | SOX11+ | 15.9 | 2.7 | 11 |
| BL-32 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-50 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-116 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-72 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-145 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-146 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-147 | EBV− | SOX11+ | 0 | 0 | NA |
| BL-148 | EBV− | SOX11+ | 0 | 0 | NA |
NA, not acquired.
The biological relevance of EBV traces may be debatable. However, traces of EBV “corrected” the EBV status of BLs almost exclusively among SOX11− but not among SOX11+ BLs, ruling out that we missed a relevant number of EBV+/SOX11+ double-positive cases by EBER, and reinforcing that SOX11 and EBV positivity are mutually exclusive in BL. Limited molecular data were available for cases tested for traces of EBV.
Oncogenic pathways regulated by SOX11 in BL cell lines
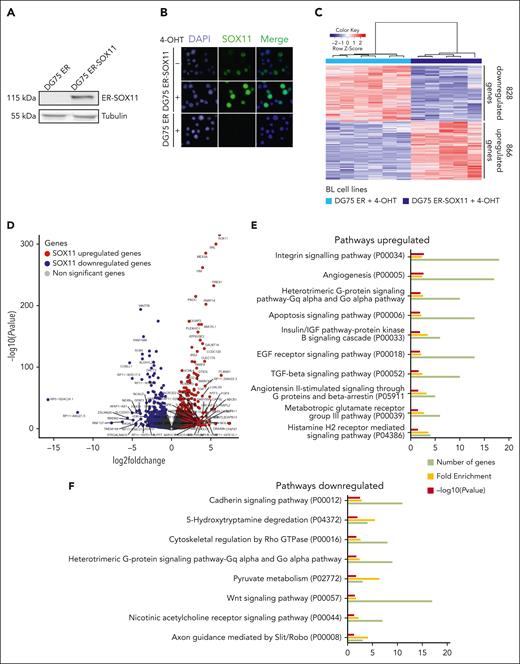
Mutual exclusivity of SOX11 and EBV status in BL was also demonstrated in the analysis of SOX11 expression in established BL cell lines (supplemental Figure 3A-B). To identify oncogenic pathways regulated by SOX11 in BL cells, we first used a SOX11− BL cell line, DG75, to ectopically express the SOX11 protein fused to the hormone binding domain of the estrogen receptor (ER-SOX11). Fusion with the ER makes SOX11 activatable upon treatment with 4-hydroxytamoxifen (4-OHT). As shown in Figure 4A-B, although expressed, the ER-SOX11 protein translocate to the nucleus only when cells are treated with 4-OHT. Because we found weak background nuclear expression in absence of 4-OHT, we decided to compare RNA-seq GEPs of DG75 ER-SOX11 and control DG75 ER cells, both treated with 4-OHT for 8 or 24 hours.
Gene expression analysis upon SOX11 overexpression in DG75-ER-SOX11 BL cell line. (A) Western blot experiment showing the levels of ER-SOX11 protein in DG75 ER-SOX11 BL cell line. DG75-ER was used as SOX11-negative control cell line, and tubulin as loading control. (B) Immunofluorescence experiments showing the nuclear localization of the SOX11 protein in DG75 ER-SOX11 cells, induced (+) or not induced (−) with 4-OHT for 24 hours. DG75 ER cell line was used as SOX11− control. DAPI (4′,6-diamidino-2-phenylindole) marks the cellular nucleus, and a merge of the 2 immunofluorescences images (DAPI and SOX11) was done. (C) Heat map illustrating the scaled expression (Z score) of 1694 DEGs (866 upregulated and 828 downregulated genes; supplemental Table 7) in DG75 ER-SOX11 compared with DG75 ER cell lines induced with 4-OHT for 8 and 24 hours, obtained by RNA-seq. Genes with an adjusted P value <.1 and absolute log2-transformed fold change of >0.65 were considered. (D) Volcano plot showing genes differentially expressed, obtained by RNA-seq, upon SOX11 overexpression in DG75 ER-SOX11 compared with DG75 ER BL cell lines treated with 4-OHT. The graph shows on the y-axis –log10(P value) and on the x-axis the log2-transformed fold change. Genes upregulated and downregulated in DG75 ER-SOX11 vs DG75 ER with an adjusted P value <.1 and log2-transformed fold change of >0.65 or less than −0.65 are colored in red and blue, respectively, and genes with an adjusted P value <.00005 and absolute log2-transformed fold change of >3 are labeled with their gene symbol. (E-F) Panther pathway enrichment analysis using DEGs (E) upregulated and (F) downregulated between DG75 ER-SOX11 and DG75 ER after 4-OHT treatment. Number of genes, fold enrichment, and –log10(P value) for each pathway are shown. Only pathways with a P value <.05 were considered.
Gene expression analysis upon SOX11 overexpression in DG75-ER-SOX11 BL cell line. (A) Western blot experiment showing the levels of ER-SOX11 protein in DG75 ER-SOX11 BL cell line. DG75-ER was used as SOX11-negative control cell line, and tubulin as loading control. (B) Immunofluorescence experiments showing the nuclear localization of the SOX11 protein in DG75 ER-SOX11 cells, induced (+) or not induced (−) with 4-OHT for 24 hours. DG75 ER cell line was used as SOX11− control. DAPI (4′,6-diamidino-2-phenylindole) marks the cellular nucleus, and a merge of the 2 immunofluorescences images (DAPI and SOX11) was done. (C) Heat map illustrating the scaled expression (Z score) of 1694 DEGs (866 upregulated and 828 downregulated genes; supplemental Table 7) in DG75 ER-SOX11 compared with DG75 ER cell lines induced with 4-OHT for 8 and 24 hours, obtained by RNA-seq. Genes with an adjusted P value <.1 and absolute log2-transformed fold change of >0.65 were considered. (D) Volcano plot showing genes differentially expressed, obtained by RNA-seq, upon SOX11 overexpression in DG75 ER-SOX11 compared with DG75 ER BL cell lines treated with 4-OHT. The graph shows on the y-axis –log10(P value) and on the x-axis the log2-transformed fold change. Genes upregulated and downregulated in DG75 ER-SOX11 vs DG75 ER with an adjusted P value <.1 and log2-transformed fold change of >0.65 or less than −0.65 are colored in red and blue, respectively, and genes with an adjusted P value <.00005 and absolute log2-transformed fold change of >3 are labeled with their gene symbol. (E-F) Panther pathway enrichment analysis using DEGs (E) upregulated and (F) downregulated between DG75 ER-SOX11 and DG75 ER after 4-OHT treatment. Number of genes, fold enrichment, and –log10(P value) for each pathway are shown. Only pathways with a P value <.05 were considered.
Principal component analysis showed that the variability between samples was significantly higher because of SOX11 overexpression (85% of the variance, principal component [PC] 1) rather than time of induction (8 or 24 hours; 4% of the variance, PC2; supplemental Figure 4A), showing that >65% of differential expressed genes (DEGs) overlapped upon SOX11 expression between 8 hours and 24 hours of 4-OHT treatment (supplemental Figure 4B).
SOX11-specific GEP in DG75 BL cell line, grouping 2 time point samples, showed 866 upregulated and 828 downregulated genes in 4-OHT–treated DG75 ER-SOX11 compared with control cells (Figure 4C-D; supplemental Table 6). Pathway enrichment analysis showed that upregulated genes in DG75 ER-SOX11 were enriched in angiogenesis, integrins, and G-protein signaling pathways, whereas downregulated genes were enriched in genes related to cadherin and Wnt signaling, among other regulatory pathways (Figure 4E).
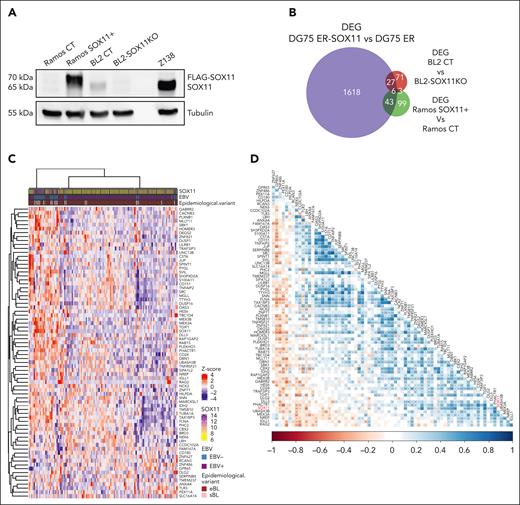
Two more cell model systems were generated to investigate the effect of SOX11 expression on the BL transcriptome: the SOX11- Ramos BL cell line ectopically overexpressing a FLAG-SOX11 protein (Ramos-SOX11+), and the SOX11+ cell line BL2 in which we knocked out the SOX11 gene using the CRISPR-associated protein 9 gene editing system (BL2-SOX11KO; Figure 5A). Changes in global gene expression were investigated in both cell systems by RNA sequencing (supplemental Figure 5A-D; supplemental Tables 7 and 8, respectively). Pathway enrichment analysis performed in Ramos cells showed enrichment of pathways in Ramos-SOX11+ similar to that observed in DG75 ER-SOX11 cell lines (supplemental Figure 5E).
SOX11–associated BL signature found in transduced cell lines is also detected in BL primary cases. (A) Western blot experiment showing the SOX11 protein levels in Ramos SOX11+ (SOX11 is FLAG-tagged) cell line, Ramos CT, BL2 CT, and BL2-SOX11KO BL cell lines. Tubulin protein was used as loading control. (B) Overlap between DEGs (adjusted P value <.1 and absolute log2-transformed fold change of >0.65) in DG75 ER-SOX11 vs DG75 ER (in purple, 1694 genes; supplemental Table 6), BL2 CT vs BL2-SOX11KO (in red, 107 genes; supplemental Table 8), and Ramos SOX11+ vs Ramos CT (in green, 151 genes; supplemental Table 7). (C) Row scaled expression (Z score) of genes from the SOX11–associated BL signature (79 DEGs overlapping between at least 2 comparisons in Figure 5B) in RNA-seq data of 117 pediatric eBLs and sBLs. K-means clustering was performed to separate samples in k = 3 groups. SOX11 mRNa expression, EBV status, and BL epidemiological variant are shown at the top panel. (D) Correlation plot between genes from the SOX11–associated BL signature in RNA-seq data of 117 pediatric eBLs and sBLs. Blue and red showed positive and negative Pearson correlation coefficients, respectively. P values from Pearson correlation are shown: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
SOX11–associated BL signature found in transduced cell lines is also detected in BL primary cases. (A) Western blot experiment showing the SOX11 protein levels in Ramos SOX11+ (SOX11 is FLAG-tagged) cell line, Ramos CT, BL2 CT, and BL2-SOX11KO BL cell lines. Tubulin protein was used as loading control. (B) Overlap between DEGs (adjusted P value <.1 and absolute log2-transformed fold change of >0.65) in DG75 ER-SOX11 vs DG75 ER (in purple, 1694 genes; supplemental Table 6), BL2 CT vs BL2-SOX11KO (in red, 107 genes; supplemental Table 8), and Ramos SOX11+ vs Ramos CT (in green, 151 genes; supplemental Table 7). (C) Row scaled expression (Z score) of genes from the SOX11–associated BL signature (79 DEGs overlapping between at least 2 comparisons in Figure 5B) in RNA-seq data of 117 pediatric eBLs and sBLs. K-means clustering was performed to separate samples in k = 3 groups. SOX11 mRNa expression, EBV status, and BL epidemiological variant are shown at the top panel. (D) Correlation plot between genes from the SOX11–associated BL signature in RNA-seq data of 117 pediatric eBLs and sBLs. Blue and red showed positive and negative Pearson correlation coefficients, respectively. P values from Pearson correlation are shown: ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
SOX11–associated BL signature
Using RNA sequencing, we overlapped the DEGs obtained between Ramos-SOX11+ and Ramos-control (CT) cell lines (supplemental Table 7); between BL2 CT and BL2-SOX11KO cell lines (supplemental Table 8), and between DG75 ER-SOX11 and DG75 ER cell lines (supplemental Table 6). In total, 79 genes commonly regulated by SOX11 in at least 2 different BL cell lines were considered to define a SOX11–associated BL signature (Figure 5B; supplemental Table 9).
We performed k-means clustering analysis in the previously published 117 pediatric BL primary cases from the BLGSP21 with RNA-seq data available, using the SOX11–associated BL signature. We observed that BL cases clustered separately according to SOX11 high and low expression levels (Figure 5C). Moreover, most of the genes included in the SOX11–associated BL signature significantly correlated between them and with SOX11 expression in this BL series (Figure 5D). Finally, by gene set enrichment analysis we observed that the SOX11–associated BL signature (supplemental Table 9) was enriched in SOX11high expression compared with SOX11low in BL cases21 (SOX11 mRNA cutoff = 10.5 log2-transformed value; supplemental Figure 6). These results suggest a similar SOX11 transcriptional activity in cell lines and primary BLs.
SOX11 functional role in MCL and BL
SOX11 directly regulates the transcription of genes involved in MCL oncogenic pathways.27 To determine whether SOX11 regulated genes in MCL were also modulated in BL, we investigated by gene set enrichment analysis the expression of SOX11-target genes identified in our previous studies in MCL cell lines28 and primary samples34 (supplemental Table 10), and in SOX11+ and SOX11− BL cell lines (DG75 ER-SOX11 vs DG75 ER; Ramos SOX11+ vs Ramos CT; BL2CT vs BL2-SOX11KO; Figure 6A). In contrast, the SOX11–associated BL signature was significantly enriched in SOX11+ compared with SOX11− MCL cell lines and primary samples (supplemental Figure 7A-B, respectively). Together, these results demonstrate that SOX11 regulates common genes in the 2 lymphoma entities.
SOX11 shares similar transcriptional roles in MCL and BL. (A) Dot plot showing at the x-axis the normalized enrichment score (NES), and at the y-axis the SOX11-target genes identified in our previously studies in MCL cell lines by chromatin immunoprecipitation (ChIP) with DNA microarray (chip; SOX11-direct targets in MCL), upregulated (SOX11 MCL signature-UP), or downregulated (SOX11 MCL signature-Down) in SOX11+ compared with SOX11− MCL cell line (Z138CT vs Z138SOX11KO) and primary samples (conventional molecular subtype [cMCL] vs nonnodal MCL subtype [nnMCL]), enriched in SOX11+ compared with SOX11− BL cell lines (DG75 ER RE-SOX11 vs DG75 ER; Ramos-SOX11+ vs Ramos CT; BL2CT vs BL2-SOX11KO). Outlined in color codes is the false discovery rate. Size of bubbles represent number of enriched genes. (B) Overlap between DEG in 4-OHT–treated DG75 ER-SOX11 BL cell line (in yellow, 1660 genes with gene symbol), upon SOX11 KO in Z138 MCL cell line (in green, 686 genes with gene symbol), and SOX11-direct target genes in MCL found by ChIP on chip in Z138 cell line (in blue, 1909 genes with gene symbol). (C) Pathway enrichment analysis on common genes between DEG in DG75 ER-SOX11 BL cells, and upon SOX11 KO in Z138 MCL cells and SOX11 targets genes obtained by ChIP on chip in Z138 MCL cells (red circle). Number of genes, fold enrichment, and –log10(P value) for each pathway are shown. (D) PLXNB1, CD24, and MEX3A relative mRNA expression (normalized to glucuronidase beta [GUSB] gene endogenous control) in BL and MCL SOX11-overexpressing cell lines (left, Ramos-SOX11+, DG75 ER-SOX11, and JVM2-SOX11+), and in SOX11-KO BL and MCL cell lines (right, BL2-SOX11KO and Z138-SOX11KO). Data are shown as mean ± standard deviation of the fold change between SOX11-overexpressing or SOX11-KO and its respective control cell values, obtained in 3 independent experiments. Statistical significance was obtained by unpaired 2-tailed t test. (E) Western blot experiments showing MEX3A and SOX11 protein levels (ER-SOX11, SOX11-FLAG, or endogenous SOX11) in BL2-CT, DG75 ER-SOX11, and Ramos-SOX11+ and their control cell lines BL2-SOX11KO CT, DG75 ER, and Ramos CT. Tubulin was used as a loading control. (F) Histograms showing CD24 protein levels analyzed by flow cytometry in SOX11− (Z138-SOX11KO and BL-SOX11KO) MCL and BL cell line models and their respective SOX1+ control cells. CD24 staining is shown in filled dark blue histograms for SOX11+ cells and in filled light-blue histograms for SOX11− cells, whereas isotype controls are shown in nonfilled and long-dashed histograms. (G) CXCR5, CCR7, and ITGB7 mRNA expression levels (log2-transformed values) in DG75 ER and DG75 ER-SOX11, obtained by RNA sequencing. (H) Relative adhesion to VCAM-1 measured as the ratio of fluorescence emission of calcein-labeled cells between those that have been attached to untreated microplate wells precoated with VCAM-1 and those attached in an unspecific way (VCAM-1 adhesion/unspecific cell adhesion in bovine serum albumin [BSA] 0.5%). Statistical significance was obtained by unpaired 2-tailed t test. ∗P < .05; ∗∗P < .01, ∗∗∗P < .001; ∗∗∗∗P < .0001.
SOX11 shares similar transcriptional roles in MCL and BL. (A) Dot plot showing at the x-axis the normalized enrichment score (NES), and at the y-axis the SOX11-target genes identified in our previously studies in MCL cell lines by chromatin immunoprecipitation (ChIP) with DNA microarray (chip; SOX11-direct targets in MCL), upregulated (SOX11 MCL signature-UP), or downregulated (SOX11 MCL signature-Down) in SOX11+ compared with SOX11− MCL cell line (Z138CT vs Z138SOX11KO) and primary samples (conventional molecular subtype [cMCL] vs nonnodal MCL subtype [nnMCL]), enriched in SOX11+ compared with SOX11− BL cell lines (DG75 ER RE-SOX11 vs DG75 ER; Ramos-SOX11+ vs Ramos CT; BL2CT vs BL2-SOX11KO). Outlined in color codes is the false discovery rate. Size of bubbles represent number of enriched genes. (B) Overlap between DEG in 4-OHT–treated DG75 ER-SOX11 BL cell line (in yellow, 1660 genes with gene symbol), upon SOX11 KO in Z138 MCL cell line (in green, 686 genes with gene symbol), and SOX11-direct target genes in MCL found by ChIP on chip in Z138 cell line (in blue, 1909 genes with gene symbol). (C) Pathway enrichment analysis on common genes between DEG in DG75 ER-SOX11 BL cells, and upon SOX11 KO in Z138 MCL cells and SOX11 targets genes obtained by ChIP on chip in Z138 MCL cells (red circle). Number of genes, fold enrichment, and –log10(P value) for each pathway are shown. (D) PLXNB1, CD24, and MEX3A relative mRNA expression (normalized to glucuronidase beta [GUSB] gene endogenous control) in BL and MCL SOX11-overexpressing cell lines (left, Ramos-SOX11+, DG75 ER-SOX11, and JVM2-SOX11+), and in SOX11-KO BL and MCL cell lines (right, BL2-SOX11KO and Z138-SOX11KO). Data are shown as mean ± standard deviation of the fold change between SOX11-overexpressing or SOX11-KO and its respective control cell values, obtained in 3 independent experiments. Statistical significance was obtained by unpaired 2-tailed t test. (E) Western blot experiments showing MEX3A and SOX11 protein levels (ER-SOX11, SOX11-FLAG, or endogenous SOX11) in BL2-CT, DG75 ER-SOX11, and Ramos-SOX11+ and their control cell lines BL2-SOX11KO CT, DG75 ER, and Ramos CT. Tubulin was used as a loading control. (F) Histograms showing CD24 protein levels analyzed by flow cytometry in SOX11− (Z138-SOX11KO and BL-SOX11KO) MCL and BL cell line models and their respective SOX1+ control cells. CD24 staining is shown in filled dark blue histograms for SOX11+ cells and in filled light-blue histograms for SOX11− cells, whereas isotype controls are shown in nonfilled and long-dashed histograms. (G) CXCR5, CCR7, and ITGB7 mRNA expression levels (log2-transformed values) in DG75 ER and DG75 ER-SOX11, obtained by RNA sequencing. (H) Relative adhesion to VCAM-1 measured as the ratio of fluorescence emission of calcein-labeled cells between those that have been attached to untreated microplate wells precoated with VCAM-1 and those attached in an unspecific way (VCAM-1 adhesion/unspecific cell adhesion in bovine serum albumin [BSA] 0.5%). Statistical significance was obtained by unpaired 2-tailed t test. ∗P < .05; ∗∗P < .01, ∗∗∗P < .001; ∗∗∗∗P < .0001.
Moreover, we observed that 185 of 1660 (11%) of the DEGs in DG75 ER-SOX11 BL cell line overlapped with those differentially expressed in Z138 SOX11+ vs Z138-SOX11KO MCL cell lines; and 134 of 1660 (8%) of the DEGs in the DG75 ER-SOX11 BL cell line overlapped with SOX11-direct target genes in the Z138 MCL cell line. Furthermore, 22 genes overlapped between the 3 comparisons. Together, these 297 genes (Figure 6B, red circle) were involved in oxidative stress, heterotrimeric G-proteins, chemokines, cytokines, integrins, angiogenesis, and platelet-derived growth factor (PDGF) signaling pathways (Figure 6C). We validated the upregulation of some of the overlapped genes, specifically the mRNA of PLXNB1, MEX3A, and CD24, and the protein levels of MEX3A and CD24 upon SOX11 overexpression and KO, in both MCL and BL transduced cell lines (Figure 6D-F). The upregulated mRNA levels of PLXNB1, CD24, and MEX3A significantly decreased, reaching similar levels as in DG75 ER control and in DG75 ER-SOX11 cells upon 4-OHT washout (supplemental Figure 8).
In BL cells, we observed that CXCR5, CCR7, and integrin-β7 (ITGB7) were significantly upregulated in 4-OHT–treated DG75 ER-SOX11 compared with DG75 ER control cells (Figure 6G). However, contrary to MCL, we did not observe a significant higher tumor cell migration toward CXCL13 or adhesion to SNKT stromal cells32 or an increase in the activation of B-cell receptor signaling pathway30 comparing SOX11+ and SOX11− BL cell line models (data not shown). Interestingly, we observed a significantly higher adhesion of SOX11+ to vascular cell adhesion molecule 1 (VCAM-1), a glycoprotein that interacts with integrin-α4β7 (ITGA4 and ITGB7) compared with SOX11− BL cells (Figure 6H).
Discussion
EBV infection is considered a crucial and early event in BL development, particularly in eBL. EBV− and EBV+ BLs exhibit distinct molecular characteristics whereas the clinical features of both groups are variable.13-18,21 SOX11 is expressed in a broad range of BLs,22,23 with a higher frequency in pediatric patients.7 Several studies have described oncogenic functions of SOX11 in MCL.28-34 However, the functional role of SOX11 in BL and its relation to EBV remains unknown.
Our results have revealed a clear negative association between SOX11 expression and EBV infection. The absence of SOX11 in EBV+ BL seems associated with the presence of the virus rather than the historical epidemiologic subtype because we also observed high SOX11 expression in the few EBV− BLs from Africa.
The IG::MYC translocation is considered the genetic hallmark of BL. EBV+ BLs mainly acquire the translocation during SHM, whereas EBV− BLs acquire it through CSR.16,18,20 Here, we add another layer of information. Among EBV− BL, we found differences in IG partners according to SOX11 expression. We observed significant differences between IG partner in MYC translocations among the 3 different groups analyzed, detecting lower proportion of IGH and higher of IGL in EBV−/SOX11+ cases. EBV−/SOX11+ BLs acquired the IG::MYC translocation during the CSR process. These data suggest that among EBV− BL, SOX11 expression is associated with 1 of the earliest events in the pathogenesis of the tumor, the occurrence of the MYC translocation.
Recently, Roco et al suggested that CSR occurs outside the germinal center earlier in the B-cell differentiation process than SHM.41 One might speculate, that in EBV−/SOX11+ BL predominant CSR-mediated IG::MYC translocations reflect this situation arising before the cell enters the germinal center. SOX11 represses BCL6 and AICDA expression in conventional MCL, possibly preventing the entrance of the tumor cell in the germinal center.29 However, no significant differences in AICDA mRNA levels were observed between EBV−/SOX11− and EBV−/SOX11+ BLs.
BL is the lymphoma entity second to MCL with most SOX11+ cases. Nevertheless, the expression levels in BL are ∼2-times lower than in MCL cases,23 suggesting that its levels in SOX11+ BL are not sufficient to fully block the entrance into the germinal center. However, confirmation of this idea needs further functional studies.
EBV+ BL cells have fewer driver gene mutations than EBV−, indicating that EBV infection may relieve the pressure toward selection of mutagenic mechanism.7,15,16,19-21 Differences in the genetic profile between EBV+ and EBV− BL cases have previously been described,14-17,21 however we have observed that EBV−/SOX11+ BLs display a distinctive mutational landscape, with significant higher frequency of mutations in SMARCA4 and ID3 than in EBV−/SOX11− and EBV+ cases. Interestingly, concomitant SMARCA4 mutations and SOX11 expression has also been observed in MCL cases.42 In addition, EBV+ BLs exhibited significant higher frequency of mutations in DDX3X and SIN3A and fewer in MYC than SOX11+ but not SOX11− BLs. These findings suggest that EBV+, EBV−/SOX11−, and EBV−/SOX11+ cases might have different oncogenic mechanisms driving their pathogenesis.
By the overlap of SOX11-mediated DEGs in 3 different BL cell lines, we obtained a SOX11-associated BL signature that consistently grouped separately SOX11+ and SOX11− BL cases. Furthermore, SOX11 overexpression in BL recapitulated, in part, the SOX11–associated transcriptional program found in MCL cells, overlapping with some of the validated pathways directly regulated by SOX11 in MCL27 such as the overexpression of PLXNB1 and CD24 involved in tumor cell migration,43,44 and MEX3A involved in the chemoresistance of colorectal cancer quiescent cells.45 However, in vitro experiments suggest that the activation of tumor cell migration32 or B-cell receptor pathways30 observed in MCL may not be so relevant in BL.
ITGB7 controls the cell homing to Peyer patches through the binding to VCAM-1.46,47 We observed a significant upregulation of ITGB7 gene expression and significantly higher adhesion to VCAM-1 in SOX11+ than in SOX11− BL cell lines. sBL, the clinical subtype that contains more SOX11+ cases, shows predominantly abdominal tumor presentation and class switch to IgA isotype,16,48 specifically seen in mucosal tissues,49 such as Peyer patches, all suggesting that BL SOX11+ cells might have a higher migration to Peyer patches, through the upregulation of ITGB7, than SOX11− BL cells. However, further studies are needed to validate this latter hypothesis.
In conclusion, SOX11 expression and EBV infection occur in alternative subsets of BL with different profiles of somatic mutations and different mechanism generating MYC translocations. The predominance of IGH class switch mediated MYC translocation in SOX11+ BL suggests an earlier development than in SOX11− tumors. Further studies are required to define the functional role of SOX11 in BL.
Acknowledgments
This work was supported by the Spanish Ministerio de Ciencia e Innovación (grant number PID2021-124048OB-100) (V.A.), cofinanced by the Sub-Directorate General for Evaluation and the European Regional Development Fund; Fondo Europeo de Desarrollo Regional (Una manera de hacer Europa); Generalitat de Catalunya Suport Grups de Recerca (Agencia de Gestió d’ajuts universitaris i de recerca [AGAUR] Senior Research Group; 2021-SGR-0129) (V.A.); and Fundació La Marató de TV3 (201901-30) (V.A.). This study was supported by the AGAUR (investigador novell-FI2018; RH041713) (M.S.-G.); the Asociación Española contra el cancer (Investigadores 2018 fellowship; INVES18042AMAD) (P.B.); the Agencia de Gestió d’ajuts universitaris i de recerca (AGAUR PERIS Salut; SLT017/20/000161) (A.D.B.); and the Agencia de Gestió d’ajuts universitaris i de recerca (AGAUR Investigo2022; 100028TC4), financed by the European Union, Next Generation European Union (M.C.-C). E.C. is an academia researcher of the Institució Catalana de Recerca i Estudis Avançats of the Generalitat de Catalunya, Centres de Recerca de Catalunya Programme/Generalitat de Catalunya. C.L. is supported by a postdoctoral Beatriu de Pinós grant from Secretaria d’Universitats i Recerca del Departament d’Empresa i Coneixement de la Generalitat de Catalunya and by Marie Sklodowska-Curie COFUND program from H2020 (2018-BP-00055). The analyses of J.R. have been supported by the Deutsche Forschungsgemeinschaft (SFB1074 project B9). The ICGC MMML-Seq consortium has been supported by the German Ministry of Science and Education in the framework of the International Cancer Genome Consortium Molecular Mechanisms of Malignant Lymphoma with Sequencing Consortium (01KU1002) and International Cancer Genome Consortium, Germany (01KU1505). The work was additionally supported by the Kinderkrebsinitiative Buchholz, Holm-Seppensen (KKI), for continuous research support (I.I., J.R., and W.K.) and for the Christoph-Schuber-Programm (I.I.) and by the clinical research unit “CATCH ALL” (KFO 5010/1) funded by the Deutsche Forschungsgemeinschaft.
Authorship
Contributions: M.S.-G. designed, performed, and interpreted all in vitro experiments, bioinformatic, and statistical analyses, and wrote the manuscript; I.I. designed and interpreted experiments, and performed bioinformatic and statistical analyses; J.R., M.M., C.L., L.L., W.K., and R.S. provided and analyzed molecular and clinical data of patients with BL; P.B., M.-L.R., A.D.B., M.C.-C., and M.M. performed in vitro experiments; M.C.S., S.T., C.B., and R.B. performed droplet digital polymerase chain reaction, immunohistochemistry, and RNAscope assay; F.N., G.C., P.J., and S.G. performed bioinformatic and statistical analyses; E.C. helped to interpret data; W.K. and V.A. designed and supervised experiments, analyzed data, and wrote the manuscript; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The institution of W.K. has received research funding from Roche, Janssen, Amgen, Takeda, Regeneron, and Incyte, not related to the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Virginia Amador, Centre Esther Koplowitz, C/Rosselló 149-153, 08036 Barcelona, Spain; email: vamador@recerca.clinic.cat.
References
Author notes
M.S.-G. and I.I. contributed equally to this study.
V.A. and W.K. are joint senior authors.
The RNA sequencing (RNA-seq) raw data used in our manuscript “BLD-2023-023242R1” have been successfully registered with the BioProject database. Our project information will be accessible at http://www.ncbi.nlm.nih.gov/bioproject/1097853 (BioProject ID: PRJNA1097853). The RNA-seq data reported in this paper will be available in the Gene Expression Omnibus database at the time of manuscript publication.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![SOX11 shares similar transcriptional roles in MCL and BL. (A) Dot plot showing at the x-axis the normalized enrichment score (NES), and at the y-axis the SOX11-target genes identified in our previously studies in MCL cell lines by chromatin immunoprecipitation (ChIP) with DNA microarray (chip; SOX11-direct targets in MCL), upregulated (SOX11 MCL signature-UP), or downregulated (SOX11 MCL signature-Down) in SOX11+ compared with SOX11− MCL cell line (Z138CT vs Z138SOX11KO) and primary samples (conventional molecular subtype [cMCL] vs nonnodal MCL subtype [nnMCL]), enriched in SOX11+ compared with SOX11− BL cell lines (DG75 ER RE-SOX11 vs DG75 ER; Ramos-SOX11+ vs Ramos CT; BL2CT vs BL2-SOX11KO). Outlined in color codes is the false discovery rate. Size of bubbles represent number of enriched genes. (B) Overlap between DEG in 4-OHT–treated DG75 ER-SOX11 BL cell line (in yellow, 1660 genes with gene symbol), upon SOX11 KO in Z138 MCL cell line (in green, 686 genes with gene symbol), and SOX11-direct target genes in MCL found by ChIP on chip in Z138 cell line (in blue, 1909 genes with gene symbol). (C) Pathway enrichment analysis on common genes between DEG in DG75 ER-SOX11 BL cells, and upon SOX11 KO in Z138 MCL cells and SOX11 targets genes obtained by ChIP on chip in Z138 MCL cells (red circle). Number of genes, fold enrichment, and –log10(P value) for each pathway are shown. (D) PLXNB1, CD24, and MEX3A relative mRNA expression (normalized to glucuronidase beta [GUSB] gene endogenous control) in BL and MCL SOX11-overexpressing cell lines (left, Ramos-SOX11+, DG75 ER-SOX11, and JVM2-SOX11+), and in SOX11-KO BL and MCL cell lines (right, BL2-SOX11KO and Z138-SOX11KO). Data are shown as mean ± standard deviation of the fold change between SOX11-overexpressing or SOX11-KO and its respective control cell values, obtained in 3 independent experiments. Statistical significance was obtained by unpaired 2-tailed t test. (E) Western blot experiments showing MEX3A and SOX11 protein levels (ER-SOX11, SOX11-FLAG, or endogenous SOX11) in BL2-CT, DG75 ER-SOX11, and Ramos-SOX11+ and their control cell lines BL2-SOX11KO CT, DG75 ER, and Ramos CT. Tubulin was used as a loading control. (F) Histograms showing CD24 protein levels analyzed by flow cytometry in SOX11− (Z138-SOX11KO and BL-SOX11KO) MCL and BL cell line models and their respective SOX1+ control cells. CD24 staining is shown in filled dark blue histograms for SOX11+ cells and in filled light-blue histograms for SOX11− cells, whereas isotype controls are shown in nonfilled and long-dashed histograms. (G) CXCR5, CCR7, and ITGB7 mRNA expression levels (log2-transformed values) in DG75 ER and DG75 ER-SOX11, obtained by RNA sequencing. (H) Relative adhesion to VCAM-1 measured as the ratio of fluorescence emission of calcein-labeled cells between those that have been attached to untreated microplate wells precoated with VCAM-1 and those attached in an unspecific way (VCAM-1 adhesion/unspecific cell adhesion in bovine serum albumin [BSA] 0.5%). Statistical significance was obtained by unpaired 2-tailed t test. ∗P < .05; ∗∗P < .01, ∗∗∗P < .001; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/2/10.1182_blood.2023023242/2/m_blood_bld-2023-023242-gr6.jpeg?Expires=1767722601&Signature=Lb94of1ov4fJZeU~LY3FxfkwV7CQnhrf6~LhLtStpkBgm0ig1DL09uJ~HzzYgQbhnFeGg1p1WxoeO-N1eZloSNAu0VBBX~4SG-IjKv7SoYqakYSlx0uLEVD-MPQXoqcS0pJ0sOTJPumz8OMM2SB5V8ET8iMkrKckiI2MtGL~EoPPlu1mRyXmYVLjggJVdBmgz7szVm7c5Z1ZG8KZQw7Reyc7WRp~EVF2Ci5g3C7fTQwdUuQcmjhgCNHWLyHMFj0lOzA3UuXroxy9eguxPHrBO6p-6JN5XHH-Gy9TZDkVFgSwIZgPjc0n4ak8gltiaP9tkzg7NR5HHM0txIsPexhaFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal