Comparison of the 2007 EORTC/ISCL and the 2022 EORTC/ISCL/USCLC blood staging guidelines for cutaneous T-cell lymphoma at a single institution reveals the newer guidelines fail to detect a subset of patients with Sézary syndrome with low blood burden.
TO THE EDITOR:
The prognostic value of blood disease burden in cutaneous T-cell lymphoma (CTCL) is well established. Although Sézary syndrome (SS) is defined as the leukemic variant of CTCL, the cutaneous presentation of mycosis fungoides (MF) may also have blood involvement.1-3 Furthermore, there is controversy regarding the distinction between MF and SS, because both entities may present with limited patch disease evolving into erythroderma and, likewise, both entities can evolve from a primarily cutaneous process into a leukemic state.4 Although the mutational signature of MF and SS are slightly different, the driving mutations in advanced disease are similar.5 Given this knowledge, the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC) incorporated blood staging and classification criteria into the TNMB (tumor, lymph node, metastasis, and blood) staging guidelines for MF/SS in 2007.6 These guidelines proposed defining B0 as ≤5% Sézary cells of the total lymphocyte population; B2 as clonal rearrangement of T-cell receptor in the blood and either (1) ≥1000 Sézary cells per μL in the blood, (2) a CD4:CD8 ratio of ≥10, or (3) ≥40% or ≥30% of CD4+CD7− or CD4+CD26− atypical lymphocytes of total lymphocytes, respectively; and B1 as patients meeting neither B0 or B2 criteria.6 In 2018, Scarisbrick et al proposed using only absolute values of CD4+CD7− or CD4+CD26− populations based on flow cytometry for B2 staging, plus a relevant clonal T-cell population.7 This proposal eliminated the CD4:CD8 ratio of ≥10, and the CD4+CD7− or CD4+CD26− percentage criteria. In 2022, in conjunction with the United States Cutaneous Lymphoma Consortium, the ISCL, EORTC, and, eventually, the National Comprehensive Cancer Network embraced the use of absolute values and updated their guidelines to reflect the recommendations previously published by Scarisbrick et al.8,9
We performed a retrospective comparative analysis of the original 2007 EORTC/ISCL guidelines and the updated 2022 EORTC/ISCL/United States Cutaneous Lymphoma Consortium (USCLC) guidelines for the classification of blood involvement applied to initial flow cytometry results on a well-characterized cohort of patients with CTCL. All patients presented to the Northwestern Medicine Cutaneous Lymphoma clinic with newly diagnosed CTCL and, as part of their staging workup, obtained a complete blood count and synchronous flow cytometry analysis before receiving any systemic therapies. Clinical data including disease course, treatments, and patient outcomes were available for the entire cohort. A total of 88 patients with CTCL were ultimately designated as being diagnosed with MF (19) or SS (69) according to disease evolution with repeated blood burden assessments and retrospective review of each case. The median age at diagnosis was 64 years (range, 27-90). The median follow-up time was 54 months (range, 1-193). Median overall survival was 67 months (range, 1-139; supplemental Table, available on the Blood website). A subset of patients had short follow-up because of rapidly progressive disease and demise. Therapy was tiered on a scale of 1-3. Tier 1 included topical therapies, tier 2 included immunomodulating systemic treatment (eg, interferon, retinoids, or methotrexate), and tier 3 included chemotherapy with/without allogeneic hematopoietic stem cell transplant (SCT).
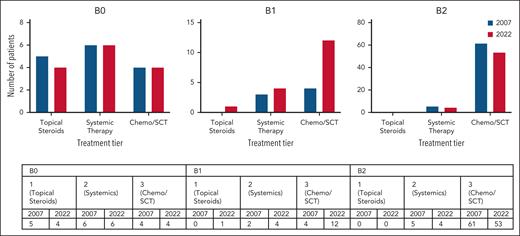
The 2007 and 2022 staging criteria were independently applied to blood flow cytometry data from initial presentation. Substantial correlation was found between the original and updated guidelines (Cohen = 0.73; 95% confidence interval, 0.596-0.874), highlighting that although there is generally agreement between the 2 staging recommendations, a small subset of patients with CTCL are staged differently. There were no differences in treatment requirements between patients staged B0/B1/B2 according to 2007 criteria and patients staged B0/B1/B2 according to 2022 criteria (Figure 1). The 2007 guidelines initially identified more patients with an ultimate diagnosis of SS, with only 4 SS cases classified as B1 at initial staging, and the remaining as B2. Conversely, the 2022 criteria initially classified 12 patients (11%) as B1, all of whom ultimately reached leukemic status. Of the 8 patients classified as B1 by only the 2022 guidelines, 7 were characterized by low atypical lymphocyte counts (<1000 cells per μL), 5 of whom also met the 2007 criterion of a CD4:CD8 ratio of ≥10. Of 8 patients, 1 solely met the CD4:CD8–ratio criterion for B2 classification, now eliminated from the 2022 proposed guidelines. These 8 patients with SS with initial B1 staging had a similarly poor prognosis as the remaining SS patients in our cohort.
Summary of patient treatment requirements according to both 2007 and 2022 blood staging criteria. More patients with 2022 B1 disease required tier 3 treatment (n = 12) compared with patients with 2007 B1 (n = 4). However, there were no significant differences in treatment requirements for 2007 B0 vs 2022 B0 (P = .96), 2007 B1 vs 2022 B1 (P = .55), and 2007 B2 vs 2022 B2 (P > .99). ECP, extracorporeal photopheresis; SCT, stem cell transplant.
Summary of patient treatment requirements according to both 2007 and 2022 blood staging criteria. More patients with 2022 B1 disease required tier 3 treatment (n = 12) compared with patients with 2007 B1 (n = 4). However, there were no significant differences in treatment requirements for 2007 B0 vs 2022 B0 (P = .96), 2007 B1 vs 2022 B1 (P = .55), and 2007 B2 vs 2022 B2 (P > .99). ECP, extracorporeal photopheresis; SCT, stem cell transplant.
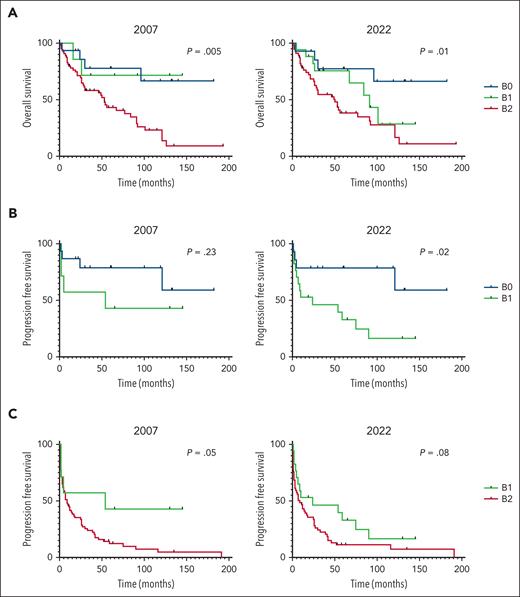
The discordance between the 2 recommendations raises questions about the respective guidelines’ prognostic ability. Both the 2007 and 2022 recommendations predict worse survival for patients with higher B stage (P = .005 and P = .01, respectively; Figure 2A). Interestingly, neither criterion distinguish B1 as its own stage. The 2022 criteria more effectively predicted progression-free survival between patients with B0 and B1 disease (P = .02) when compared with the 2007 criteria (P = .23; Figure 2B). Conversely, the 2007 guidelines better predicted progression-free survival between patients with B1 and B2 disease (P = .05) when compared with the 2022 guidelines (P = .08; Figure 2C).
Kaplan-Meier analyses of the overall survival and progression-free survival for patients based on 2007 and 2022 staging guidelines. (A) Overall survival for stages B0, B1, and B2. (B) Progression-free survival (PFS) for stages B0 and B1. (C) PFS for stages B1 and B2.
Kaplan-Meier analyses of the overall survival and progression-free survival for patients based on 2007 and 2022 staging guidelines. (A) Overall survival for stages B0, B1, and B2. (B) Progression-free survival (PFS) for stages B0 and B1. (C) PFS for stages B1 and B2.
Our analyses emphasize the importance of incorporating percentages of CD4+CD26− and CD4+CD7− atypical lymphocytes in blood staging for MF/SS, as opposed to solely relying on absolute values. This is especially important for patients with a low number of circulating abnormal cells and lymphopenia. Although a clinically significant proportion of lymphocytes may exhibit an abnormal phenotype, and therefore would fit B2 criteria using the original percentage threshold, their lymphopenic state deceptively ensures that they remain below the absolute value threshold of 1000 cells per μL, endorsed by the updated criteria. Inclusion of the percentage of atypical cells in the blood assessment likely contributes to the original 2007 guidelines’ improved ability to predict disease progression between patients with B1 and B2 disease. Our analysis also reveals that the inclusion of CD4:CD8 ratio allows the 2007 guidelines to earlier identify patients with eventual leukemic evolution. A high CD4:CD8 ratio has been found to be prevalent in patients with SS and is associated with worse prognosis. Thus, including this criterion may assist with both diagnostic and prognostic purposes.10
A shortcoming of the 2007 guidelines is the lack of significant outcome distinction between patients with B0 and B1 disease. Conversely, the 2022 guidelines effectively distinguish between patients with B0 and B1 disease but did not effectively distinguish patients with B1 and B2 disease. Neither guideline effectively distinguishes B1 as its own category, raising the question as to whether stage B1 should be redefined. If the 2022 guidelines prevail in our daily practice, we should assume that a B1 blood classification is practically prognostically the same as B2.
In summary, a significant subset of patients with MF/SS patients with eventual leukemic evolution were originally classified as B1 by the 2022 guidelines, whereas most of these cases were classified at the time of diagnosis as B2 under the 2007 criteria. Although both criteria predict a worse prognosis for patients with higher blood burden, neither has succeeded at defining an independent B1 category with intermediate prognosis. Reconsideration of the latest 2022 guidelines with inclusion of percentages of CD4+CD7− and CD4+CD26− populations and CD4:CD8 ratio of ≥10 in addition to absolute value thresholds may optimize guideline sensitivity and specificity, with a more accurate prognostic determination for patients with CTCL with blood involvement.
Ethical approval was obtained from the Northwestern University Institutional Review Board (STU00200981). Patients who provided consent were enrolled at the Northwestern University Cutaneous Lymphoma clinic in compliance with the Declaration of Helsinki.
Authorship
Contribution: L.P.C., P.F.T., D.R.P., M.E.M.-E., M.J.H., Y.P., W.Q.N., T.L.G., L.A., and R.F. performed research and curated data; P.F.T., L.F., and K.L.W. contributed analytical tools to the manuscript; L.P.C., D.R.P., and M.E.M.-E. analyzed data; L.P.C. and J.G. wrote the original draft of the manuscript; X.A.Z., K.L.W., L.F., P.F.T., D.R.P., M.E.M.-E., M.J.H., Y.P., W.Q.N., T.L.G., L.A., and R.F. reviewed and edited the original draft of the manuscript; and all authors contributed to the manuscript and approved the submitted version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joan Guitart, Department of Dermatology, Northwestern University, 676 N St Clair St, Suite 1765, Chicago, IL 60611; email: jguitart@nm.org.
References
Author notes
Data are available on request from the corresponding author, Joan Guitart (jguitart@nm.org).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal