Key Points
Epcoritamab, a CD3×CD20 bispecific antibody, plus GemOx yields deep, durable responses in second-line or later ASCT-ineligible R/R DLBCL.
Higher ORR and CR rate in less heavily pretreated patients suggest that this regimen, as an earlier treatment, may improve outcomes.
Visual Abstract
Patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) have poor outcomes (complete response [CR] rates with standard salvage therapy gemcitabine plus oxaliplatin [GemOx], ∼30%; median overall survival [OS], 10 to 13 months). Patients with refractory disease fare worse (CR rate with salvage therapy, 7%; median OS, 6 months). Epcoritamab, a CD3×CD20 bispecific antibody approved for R/R DLBCL after ≥2 therapy lines, has shown promising safety and efficacy in various combinations. We report results from the phase 1b/2 EPCORE NHL-2 trial evaluating epcoritamab plus GemOx in autologous stem cell transplant (ASCT)-ineligible R/R DLBCL. Patients received 48 mg subcutaneous epcoritamab after 2 step-up doses until progression or unacceptable toxicity; GemOx was given once every 2 weeks for 8 doses. The primary end point was overall response rate (ORR). As of 15 December 2023, 103 patients were enrolled (median follow-up, 13.2 months; median age, 72 years). Patients had challenging-to-treat disease: ≥2 prior therapy lines, 62%; prior chimeric antigen receptor T-cell therapy, 28%; primary refractory disease, 52%; refractory to last therapy, 70%. ORR and CR rate were 85% and 61%, respectively. Median duration of CR and OS were 23.6 and 21.6 months, respectively. Common treatment-emergent adverse events were cytopenias and cytokine release syndrome (CRS). CRS events had predictable timing, were primarily low grade (52% overall, 1% grade 3), and resolved without leading to discontinuation. Epcoritamab plus GemOx yielded deep, durable responses and favorable long-term outcomes in ASCT-ineligible R/R DLBCL. This trial was registered at www.clinicaltrials.gov as #NCT04663347.
Introduction
Diffuse large B-cell lymphoma (DLBCL), an aggressive subtype of lymphoma, is the most frequently diagnosed non-Hodgkin lymphoma (NHL), accounting for ∼30% of NHL.1 Patients with relapsed or refractory (R/R) disease after first-line treatment who are unable to undergo high-dose therapy followed by autologous stem cell transplant (ASCT) and those for whom ASCT has failed have dismal outcomes and limited treatment options; patients with treatment-refractory disease do especially poorly, with overall response rates (ORRs) and complete response (CR) rates of 26% and 7% to subsequent therapies, respectively, and a median overall survival (OS) of 6 months.2
Gemcitabine plus oxaliplatin (GemOx) is commonly used with rituximab (R-GemOx) as an immunochemotherapy regimen in patients who are ineligible for ASCT.3 However, outcomes are suboptimal, with a CR rate of 33%, median progression-free survival (PFS) of 5 months, and median OS of 10 months (7 months in older patient populations).3 In recently published data, R-GemOx treatment resulted in a median PFS of 3.6 months and a median OS of 12.9 months.4
Current outcomes are suboptimal in R/R DLBCL irrespective of treatment modality. Durable remissions can be achieved with first-line therapy by combining mechanistically distinct therapies to overcome cross-resistance, and similar approaches are needed to achieve durable remissions with salvage therapy. Although chimeric antigen receptor (CAR) T-cell therapies have shown long-term efficacy in R/R DLBCL,5 their utility is limited by manufacturing timelines, accessibility, logistical challenges, and the need for relatively fit patients.6-9 T-cell–engaging antibodies are an emerging treatment class that offers an off-the-shelf option with promising efficacy and reduced cytokine release syndrome (CRS) and neurotoxicity.9
Epcoritamab, a CD3×CD20 bispecific antibody, is approved for the treatment of adults with R/R DLBCL or follicular lymphoma after ≥2 lines of systemic therapy.10-13 The pivotal EPCORE NHL-1 phase 2 trial showed that epcoritamab demonstrated deep, durable responses with manageable safety in patients with third-line or later R/R LBCL.14 Long-term follow-up beyond 2.5 years showed ORR and CR rate of 63% and 40%, respectively, for patients with LBCL. CRs were durable, with 62% of patients with CR remaining in remission at 24 months.15 Epcoritamab is a distinct bispecific antibody because it is administered as a low-volume subcutaneous injection and does not require bridging therapy or debulking before treatment initiation; this allows for rapid T-cell engagement and killing of malignant CD20+ B cells.16,17 In clinical trials, epcoritamab has shown encouraging efficacy and manageable safety in combination with standard treatments, including chemotherapies.18-20
EPCORE NHL-2 is a phase 1b/2 trial of epcoritamab treatment in combination with standard therapies for patients with CD20+ NHL. Here, we report efficacy and safety results from arm 5 of the EPCORE NHL-2 trial, which evaluated epcoritamab plus GemOx for patients with R/R DLBCL who were not eligible for ASCT because of age, performance status, comorbidities, or failure of prior ASCT.
Methods
Study design and patients
EPCORE NHL-2 is a phase 1b/2, open-label, multicenter trial in patients with CD20+ NHL (EPCORE NHL-2; GCT3013-02; ClinicalTrials.gov identifier: NCT04663347). The trial has 2 parts: dose escalation and expansion.
In arm 5, adult patients were enrolled with the following key inclusion criteria: Eastern Cooperative Oncology Group performance status of 0 to 2; documented CD20+ DLBCL that was R/R to ≥1 prior therapy and de novo or histologically transformed from follicular or nodal marginal zone lymphoma according to 2016 World Health Organization classification21 (DLBCL, not otherwise specified; double- or triple-hit lymphoma with DLBCL morphology [high-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 translocations]; follicular lymphoma grade 3B; or T-cell/histiocyte-rich DLBCL); and previous ASCT failure or ineligibility for ASCT because of age, performance status, comorbidities, or insufficient response to prior treatment. Eligible patients had measurable disease, had adequate organ function, and were eligible to receive GemOx. Patients were not enrolled if they had active infections requiring systemic therapy; COVID-19 tests were not required at screening. Patients were considered to have relapsed disease if they had an initial response and experienced progression at least 6 months after last treatment. Patients were considered to have refractory disease if they experienced progression or stable disease as best overall response during therapy or progression within 6 months of completion of therapy.
Patients were treated with subcutaneous epcoritamab in 28-day cycles as follows: once weekly in cycles 1 to 3, once every 2 weeks in cycles 4 to 9, and once every 4 weeks in cycles 10 and beyond until disease progression or unacceptable toxicity.
Step-up dosing, given in cycle 1, included 2 step-up doses (0.16 mg on day 1 and 0.8 mg on day 8), followed by full doses on days 15 and beyond. In the dose-escalation part, patients were treated with either 24-mg or 48-mg full doses. In the expansion part, all patients were treated with the recommended phase 2 dose of 48 mg.22 Patients were required to have ≥7 days between epcoritamab administrations. A full repriming cycle (weekly schedule of priming, intermediate, and 2 full doses) was required if an intermediate dose was delayed by >1 day, a first full dose was delayed by >7 days, or the interval between full doses was >6 weeks. Once repriming was completed, dosing resumed at day 1 of the next planned cycle.
GemOx (IV gemcitabine, 1000 mg/m2; IV oxaliplatin, 100 mg/m2) was administered every 2 weeks for the first 4 cycles (28 days each; 8 total doses, 2 per cycle). Herein, we describe results from patients in the 48-mg epcoritamab treatment groups of dose escalation and expansion.
Prophylaxis for CRS in cycle 1 included prednisolone 100 mg or equivalent (oral or IV) 30 to 120 minutes before epcoritamab dosing (once daily on days 1 to 4 for the priming dose; once daily on days 8 to 11 for the intermediate dose; once daily on days 15 to 18 for the first full dose; and once daily on days 22 to 25 for the second full dose). Additionally, oral or IV diphenhydramine 50 mg (or equivalent) and oral acetaminophen 650 to 1000 mg were administered once daily on days 1, 8, 15, and 22 of cycle 1. Patients were required to be hospitalized for the first full dose of epcoritamab (on day 15 of cycle 1 and during repriming cycles) and for 24 hours after administration.
End points and assessments
The protocol-specified primary end point of the expansion part was ORR in accordance with Lugano criteria.23 Key secondary end points included CR rate, time to response, PFS, duration of response (DOR), and duration of CR (DOCR), all assessed by independent review committee (IRC) per Lugano criteria, and OS. Safety end points included adverse events (AEs) and laboratory values, including immunoglobulin levels.
Efficacy was evaluated by positron emission tomography–computed tomography imaging obtained at screening, at 6, 12, 18, 24, 36, and 48 weeks, and every 24 weeks thereafter, until disease progression. Exploratory minimal residual disease (MRD) analyses were performed with plasma circulating tumor DNA samples, including a cycle 3 day 1 landmark analysis, using the AVENIO Oncology Assay Non-Hodgkin Lymphoma Test (AVENIO; Roche Sequencing Solutions, Pleasanton, CA).
AEs were classified using Medical Dictionary for Regulatory Activities version 26.1, and severity was graded using National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. CRS and immune effector cell–associated neurotoxicity syndrome (ICANS) were graded using American Society for Transplantation and Cellular Therapy criteria,24 and clinical tumor lysis syndrome was graded using Cairo-Bishop criteria.25 The relationship between AEs and treatment was determined by the investigator. Tumor biopsy was assessed at screening. Blood immunophenotyping and plasma cytokines were measured using flow cytometry and the Meso Scale Discovery assay (Rockville, MD), respectively. Immunoglobulin levels were assessed locally at screening and day 1 of each cycle (predose).
Statistical analysis
There was no formal hypothesis testing in this single-arm trial. A sample size of 100 patients receiving epcoritamab 48 mg provided at least an 82% chance of detecting a 15% improvement in ORR in patients with R/R DLBCL when epcoritamab is added to GemOx, assuming the ORR is 46% for GemOx alone and the 2-sided α is 5%.
Efficacy and safety analyses were conducted in the full analysis population, comprising patients who received at least 1 dose of trial drug. Efficacy assessments were also performed in prespecified subgroups based on best overall response and characteristics. MRD analyses were conducted in the MRD-evaluable population, comprising patients with at least 1 baseline or on-treatment MRD result and MRD not negative at baseline. Continuous data were summarized descriptively, categorical data were summarized using frequency and percentage, and time-to-event data were summarized using the Kaplan-Meier method. Data were analyzed using SAS software version 9.4 or higher (SAS Institute, Inc, Cary, NC).
ORR, based on response by IRC and investigator assessments, was defined as the percentage of patients with a best overall response of CR or partial response (PR). Corresponding exact 95% confidence intervals (CIs) for ORR and CR rate were calculated using the Clopper-Pearson method.
PFS was defined as time from cycle 1 day 1 to first documented progression or death due to any cause. OS was defined as time from cycle 1 day 1 to death due to any cause. DOR was defined as time from first CR or PR to first documented progression or death due to any cause. DOCR was defined as time from first CR to first documented progression or death due to any cause. Date of disease progression was defined as the earliest date of documented progression after which there was no CR or PR. PFS and DOR were censored at the date of last disease assessment or before the date of subsequent antilymphoma therapy for patients who did not have progression and remained alive. If a patient was not known to have died, then OS was censored at the latest date that the patient was known to be alive. A sensitivity analysis for OS censored patients at the date of last disease assessment before death due to confirmed COVID-19.
Ethics
Site-specific institutional review boards or institutional or central ethics committees approved the protocol before study initiation. The study was conducted in accordance with the International Council for Harmonization E6(R2) guidelines on good clinical practice and the principles of the Declaration of Helsinki. Before enrollment, all patients reviewed and signed informed consent forms.
Results
Study population and exposure
From 25 January 2021 to 15 December 2023, 103 patients with ASCT-ineligible R/R DLBCL were treated with epcoritamab plus GemOx (supplemental Figure 1, available on the Blood website).
The median age was 72 years, with 35% of patients aged ≥75 years. Patients had a median of 2 prior lines of therapy (pLOT; range, 1-6), 38% of patients had 1 pLOT, and 62% had ≥2 pLOT. Overall, 19% had bulky disease (>10 cm) by IRC assessment, 23% had transformed DLBCL, 6 of 58 patients (10%) with evaluable tissue had double- or triple-hit lymphoma by central laboratory assessment, 61% had Ann Arbor stage IV disease, 52% had primary refractory disease, 70% had disease refractory to their last systemic therapy, 10% had prior ASCT, and 28% had prior CAR T-cell therapy. Almost all patients enrolled were from Europe (72%) or North America (27%). Demographic and baseline clinical characteristics in the overall population are shown in Table 1 (see supplemental Table 1 for patients with CR and patients with 1 pLOT). The primary reasons patients were ineligible for ASCT were age (56%), performance status (8%), prior transplant (8%), comorbidities (5%), and other reasons (22%, most of which were inadequate response to prior therapy).
Demographic and baseline clinical characteristics in the overall population
| Characteristic . | Overall N = 103 . |
|---|---|
| Age, median (range), y | 72 (20-87) |
| Age group, y, n (%) | |
| <65 | 28 (27.2) |
| 65 to <75 | 39 (37.9) |
| ≥75 | 36 (35.0) |
| Sex at birth, n (%) | |
| Male | 57 (55.3) |
| Female | 46 (44.7) |
| Race, n (%) | |
| White | 78 (75.7) |
| Black or African American | 4 (3.9) |
| Asian | 4 (3.9) |
| Other | 1 (1.0) |
| Not reported | 16 (15.5) |
| Ethnicity,∗n (%) | |
| Hispanic or Latino | 3 (2.9) |
| Not Hispanic or Latino | 22 (21.4) |
| Not reported | 3 (2.9) |
| Missing | 75 (72.8) |
| Region, n (%) | |
| Europe | 74 (71.8) |
| North America | 28 (27.2) |
| Australia | 1 (1.0) |
| ECOG performance status, n (%) | |
| 0 | 33 (32.0) |
| 1 | 57 (55.3) |
| 2 | 13 (12.6) |
| IPI, n (%) | |
| <3 | 38 (36.9) |
| ≥3 | 65 (63.1) |
| Bulky disease by IRC assessment,†n (%) | |
| <7 cm (nonbulky disease) | 62 (60.2) |
| 7 to 10 cm | 18 (17.5) |
| >10 cm | 20 (19.4) |
| DLBCL type,‡n (%) | |
| De novo | 75 (72.8) |
| Transformed | 24 (23.3) |
| Double- or triple-hit lymphoma (MYC and BCL2 and/or BCL6 rearrangement) by central laboratory,§ n/n (%) | 6/58 (10.3) |
| DLBCL cell of origin,||n (%) | |
| Activated B cell/non–germinal center B cell | 43 (41.7) |
| Germinal center B cell | 41 (39.8) |
| Unknown | 19 (18.4) |
| Ann Arbor stage, n (%) | |
| I or II | 22 (21.4) |
| III | 18 (17.5) |
| IV | 63 (61.2) |
| Time from initial diagnosis to first dose of epcoritamab, median (range), mo | 13.2 (0.6-177.7) |
| Time from end of last therapy to first dose of epcoritamab, median (range), mo | 4.6 (0.6-98.9) |
| Prior lines of antilymphoma therapy, median (range) | 2 (1-6) |
| Prior lines of antilymphoma therapy, n (%) | |
| 1 | 39 (37.9) |
| 2 | 27 (26.2) |
| ≥3 | 37 (35.9) |
| Primary refractory disease,¶,# n (%) | 54 (52.4) |
| Refractory to last systemic therapy,# n (%) | 72 (69.9) |
| Refractory to ≥2 consecutive lines of therapy,# n (%) | 38 (36.9) |
| Prior ASCT, n (%) | 10 (9.7) |
| Relapsed ≤12 mo after ASCT, n/n (%) | 5/10 (50.0) |
| Prior CAR T-cell therapy, n (%) | 29 (28.2) |
| Characteristic . | Overall N = 103 . |
|---|---|
| Age, median (range), y | 72 (20-87) |
| Age group, y, n (%) | |
| <65 | 28 (27.2) |
| 65 to <75 | 39 (37.9) |
| ≥75 | 36 (35.0) |
| Sex at birth, n (%) | |
| Male | 57 (55.3) |
| Female | 46 (44.7) |
| Race, n (%) | |
| White | 78 (75.7) |
| Black or African American | 4 (3.9) |
| Asian | 4 (3.9) |
| Other | 1 (1.0) |
| Not reported | 16 (15.5) |
| Ethnicity,∗n (%) | |
| Hispanic or Latino | 3 (2.9) |
| Not Hispanic or Latino | 22 (21.4) |
| Not reported | 3 (2.9) |
| Missing | 75 (72.8) |
| Region, n (%) | |
| Europe | 74 (71.8) |
| North America | 28 (27.2) |
| Australia | 1 (1.0) |
| ECOG performance status, n (%) | |
| 0 | 33 (32.0) |
| 1 | 57 (55.3) |
| 2 | 13 (12.6) |
| IPI, n (%) | |
| <3 | 38 (36.9) |
| ≥3 | 65 (63.1) |
| Bulky disease by IRC assessment,†n (%) | |
| <7 cm (nonbulky disease) | 62 (60.2) |
| 7 to 10 cm | 18 (17.5) |
| >10 cm | 20 (19.4) |
| DLBCL type,‡n (%) | |
| De novo | 75 (72.8) |
| Transformed | 24 (23.3) |
| Double- or triple-hit lymphoma (MYC and BCL2 and/or BCL6 rearrangement) by central laboratory,§ n/n (%) | 6/58 (10.3) |
| DLBCL cell of origin,||n (%) | |
| Activated B cell/non–germinal center B cell | 43 (41.7) |
| Germinal center B cell | 41 (39.8) |
| Unknown | 19 (18.4) |
| Ann Arbor stage, n (%) | |
| I or II | 22 (21.4) |
| III | 18 (17.5) |
| IV | 63 (61.2) |
| Time from initial diagnosis to first dose of epcoritamab, median (range), mo | 13.2 (0.6-177.7) |
| Time from end of last therapy to first dose of epcoritamab, median (range), mo | 4.6 (0.6-98.9) |
| Prior lines of antilymphoma therapy, median (range) | 2 (1-6) |
| Prior lines of antilymphoma therapy, n (%) | |
| 1 | 39 (37.9) |
| 2 | 27 (26.2) |
| ≥3 | 37 (35.9) |
| Primary refractory disease,¶,# n (%) | 54 (52.4) |
| Refractory to last systemic therapy,# n (%) | 72 (69.9) |
| Refractory to ≥2 consecutive lines of therapy,# n (%) | 38 (36.9) |
| Prior ASCT, n (%) | 10 (9.7) |
| Relapsed ≤12 mo after ASCT, n/n (%) | 5/10 (50.0) |
| Prior CAR T-cell therapy, n (%) | 29 (28.2) |
ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index.
Ethnicity was not reported for patients enrolled outside of the United States.
A total of 12 patients had bulky disease of >10 cm by investigator assessment. Bulky disease status by IRC was missing for 3 patients.
De novo vs transformed status was missing for 4 patients.
MYC and BCL2 and/or BCL6 rearrangement by central laboratory was not assessed in 45 patients.
DLBCL cell of origin was determined by local laboratory.
Primary refractory disease is defined as disease that is refractory to first-line antilymphoma therapy.
Refractory disease is defined as disease progression or stable disease as best overall response during therapy or progression within 6 months of completion of therapy.
Treatment was ongoing in 44 patients (43%) at the time of data cutoff (supplemental Table 2). A total of 59 patients (57%) discontinued trial treatment; 32 (31%) discontinued because of progressive disease; at the time of progressive disease, all but 2 patients were on epcoritamab treatment. There were 20 patients (19%) who discontinued because of AEs (COVID-19/COVID-19 pneumonia in 6 patients; pneumonia in 2 patients; aspergillosis, enterocolitis, Escherichia coli sepsis, hydrocephalus, ICANS, multiple organ dysfunction syndrome, small intestinal perforation, and subarachnoid hemorrhage in 1 patient each; 1 patient with myelitis, hemiparesis, urinary tract infection, and fall; 1 patient with pneumonia, chronic sinusitis, and respiratory tract infection; 1 patient with pneumonia and hypoxia; and 1 patient with an unspecified AE). Two patients discontinued treatment because of AEs (ICANS and pneumonia, n = 1 each) while in CR and remained in remission at data cutoff (duration of CRs after treatment discontinuation: 18 and 32 weeks, respectively). All but 1 patient received at least 1 full dose of epcoritamab. Patients initiated a median of 8 epcoritamab cycles (range, 1-37) and 4 GemOx cycles (range, 1-4). Median duration of epcoritamab treatment was 8.3 months (range, 0.3-33.2). Median relative dose intensities of gemcitabine and oxaliplatin were 80% and 78%, respectively (previous studies reported medians ranging from 73% to 93%)3,26; treatment-emergent AEs (TEAEs) led to GemOx dose modification in 88 patients (85%).
Efficacy
Median study follow-up was 13.2 months (range, 1.0-34.6). Best overall response was consistent between IRC and investigator assessments; ORR and CR rate were 85% and 61%, respectively, by IRC assessment and 80% and 58%, respectively, by investigator assessment. Three patients had PRs that converted to CRs after week 24, and 3 patients with stable disease had a PR or CR after week 12. A summary of response in the overall population by IRC and investigator assessments is shown in Table 2.
Summary of response in the overall population by IRC and investigator assessments
| . | IRC assessment N = 103 . | Investigator assessment N = 103 . |
|---|---|---|
| ORR, n (%) | 88 (85.4) | 82 (79.6) |
| CR | 63 (61.2) | 60 (58.3) |
| PR | 25 (24.3) | 22 (21.4) |
| Stable disease, n (%) | 3 (2.9) | 7 (6.8) |
| Progressive disease, n (%) | 8 (7.8) | 9 (8.7) |
| Not evaluable, n (%) | 4 (3.9) | 5 (4.9) |
| Time to response, median (range), mo | 1.5 (0.9-3.0) | 1.5 (0.9-11.1) |
| Time to CR, median (range), mo | 2.6 (1.3-22.1) | 1.7 (1.3-10.7) |
| . | IRC assessment N = 103 . | Investigator assessment N = 103 . |
|---|---|---|
| ORR, n (%) | 88 (85.4) | 82 (79.6) |
| CR | 63 (61.2) | 60 (58.3) |
| PR | 25 (24.3) | 22 (21.4) |
| Stable disease, n (%) | 3 (2.9) | 7 (6.8) |
| Progressive disease, n (%) | 8 (7.8) | 9 (8.7) |
| Not evaluable, n (%) | 4 (3.9) | 5 (4.9) |
| Time to response, median (range), mo | 1.5 (0.9-3.0) | 1.5 (0.9-11.1) |
| Time to CR, median (range), mo | 2.6 (1.3-22.1) | 1.7 (1.3-10.7) |
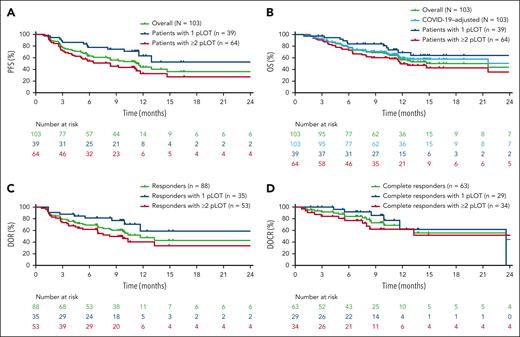
Efficacy results by IRC assessment in the overall population, in patients with CR, and by pLOT are shown in Table 3. Median PFS in the overall population and among patients with CR was 11.2 (95% CI, 8.0-14.7) and 26.7 (95% CI, 11.7 to not reached [NR]) months, respectively (Figure 1A; supplemental Figure 2A). PFS was greater in patients with 1 pLOT than those with ≥2 pLOT. Among patients with CR and patients with 1 pLOT, an estimated 68% and 63%, respectively, remained progression free at 12 months. There were minimal differences in PFS for patients who had de novo vs transformed DLBCL, with an estimated 44% of patients remaining progression free at 12 months, regardless of DLBCL type.
Summary of efficacy results by IRC assessment in the overall population, in patients with CR, and by pLOT
| . | Overall N = 103 . | Patients with CR by IRC, n = 63 . | Patients with 1 pLOT, n = 39 . | Patients with ≥2 pLOT, n = 64 . |
|---|---|---|---|---|
| Time to response, median (range), mo | 1.5 (0.9-3.0) | 1.4 (0.9-3.0) | 1.5 (1.1-2.8) | 1.4 (0.9-3.0) |
| Time to CR, median (range), mo | 2.6 (1.3-22.1) | 2.6 (1.3-22.1) | 1.6 (1.3-22.1) | 2.8 (1.4-11.2) |
| Patients remaining in response at 12 mo,∗ % | 47.6 (32.8-61.1) | 63.5 (43.5-78.1) | 58.9 (29.5-79.5) | 40.4 (23.9-56.3) |
| Patients remaining in CR at 12 mo,∗ % | 62.6 (42.3-77.5) | 62.6 (42.3-77.5) | 61.7 (24.5-84.7) | 62.1 (39.9-78.1) |
| PFS at 12 mo,∗ % | 44.0 (31.7-55.5) | 68.5 (50.0-81.3) | 63.2 (40.1-79.4) | 32.8 (19.0-47.2) |
| OS at 12 mo,∗ % | 56.6 (45.5-66.3) | 84.4 (70.8-92.0) | 69.1 (49.3-82.4) | 49.6 (36.0-61.8) |
| . | Overall N = 103 . | Patients with CR by IRC, n = 63 . | Patients with 1 pLOT, n = 39 . | Patients with ≥2 pLOT, n = 64 . |
|---|---|---|---|---|
| Time to response, median (range), mo | 1.5 (0.9-3.0) | 1.4 (0.9-3.0) | 1.5 (1.1-2.8) | 1.4 (0.9-3.0) |
| Time to CR, median (range), mo | 2.6 (1.3-22.1) | 2.6 (1.3-22.1) | 1.6 (1.3-22.1) | 2.8 (1.4-11.2) |
| Patients remaining in response at 12 mo,∗ % | 47.6 (32.8-61.1) | 63.5 (43.5-78.1) | 58.9 (29.5-79.5) | 40.4 (23.9-56.3) |
| Patients remaining in CR at 12 mo,∗ % | 62.6 (42.3-77.5) | 62.6 (42.3-77.5) | 61.7 (24.5-84.7) | 62.1 (39.9-78.1) |
| PFS at 12 mo,∗ % | 44.0 (31.7-55.5) | 68.5 (50.0-81.3) | 63.2 (40.1-79.4) | 32.8 (19.0-47.2) |
| OS at 12 mo,∗ % | 56.6 (45.5-66.3) | 84.4 (70.8-92.0) | 69.1 (49.3-82.4) | 49.6 (36.0-61.8) |
Kaplan-Meier estimates are shown with 95% CIs.
Kaplan-Meier curves of efficacy outcomes. Kaplan-Meier curves by IRC assessment for PFS (A), OS (B), DOR (C), and DOCR (D) in the overall population and by pLOT. PFS was defined as time from cycle 1 day 1 to first documented progression or death due to any cause. OS was defined as time from cycle 1 day 1 to death due to any cause. A sensitivity analysis for OS censored patients at the date of last disease assessment before death due to confirmed COVID-19. DOR was defined as time from first CR or PR to first documented progression or death due to any cause. DOCR was defined as time from first CR to first documented progression or death due to any cause.
Kaplan-Meier curves of efficacy outcomes. Kaplan-Meier curves by IRC assessment for PFS (A), OS (B), DOR (C), and DOCR (D) in the overall population and by pLOT. PFS was defined as time from cycle 1 day 1 to first documented progression or death due to any cause. OS was defined as time from cycle 1 day 1 to death due to any cause. A sensitivity analysis for OS censored patients at the date of last disease assessment before death due to confirmed COVID-19. DOR was defined as time from first CR or PR to first documented progression or death due to any cause. DOCR was defined as time from first CR to first documented progression or death due to any cause.
Median OS was 21.6 months (95% CI, 11.6 to NR) in the overall population and NR (95% CI, NR to NR) in patients with CR (Figure 1B; supplemental Figure 2B). Among patients with CR and patients with 1 pLOT, an estimated 84% and 69%, respectively, remained alive at 12 months. Kaplan-Meier curves by IRC assessment for PFS and OS in patients with CR overall and by pLOT are shown in supplemental Figure 2.
Median time to response and CR were 1.5 and 2.6 months, respectively, in the overall population (Table 2). Median DOR in the overall population was 11.7 months (Figure 1C). A higher percentage of responders with 1 pLOT remained in response at 12 months than patients with ≥2 pLOT (Table 3). Median DOCR was 23.6 months (95% CI, 11.7 to NR) overall (Figure 1D). Twelve-month estimates showed that ∼60% of complete responders remained in CR regardless of whether they had 1 pLOT, ≥2 pLOT, de novo DLBCL, or transformed DLBCL.
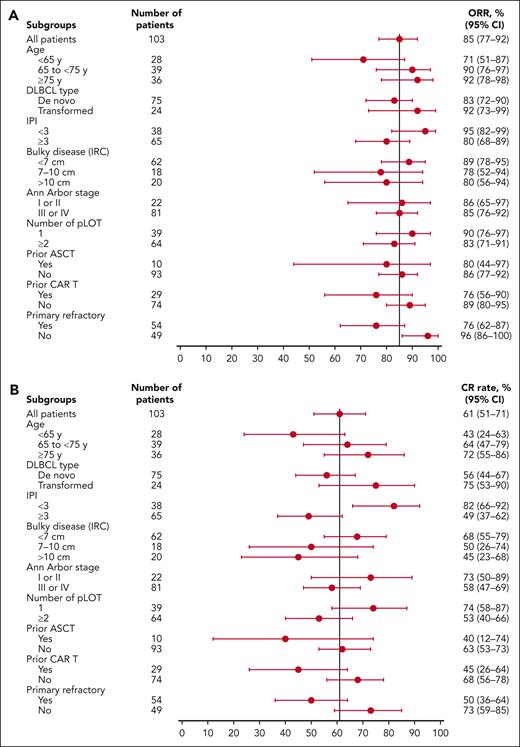
Subgroup analyses showed high CR rates: 74% in patients with 1 pLOT vs 53% in patients with ≥2 pLOT and 68% in CAR T-cell therapy–naive patients vs 45% in patients with prior CAR T-cell therapy. Among 6 patients with double- or triple-hit lymphoma, 3 (50%) had a CR and median DOCR was NR (95% CI, NR to NR). High CR rates were seen regardless of whether patients had transformed or de novo disease. ORRs and CR rates in subgroups are shown in Figure 2.
Response rates by subgroup. ORRs (A) and CR rates (B) by IRC assessment in subgroups. IPI, International Prognostic Index.
Response rates by subgroup. ORRs (A) and CR rates (B) by IRC assessment in subgroups. IPI, International Prognostic Index.
An analysis of PFS, OS, and DOCR by region showed consistent PFS, OS, and DOCR in patients from North America and patients from Europe; 12-month estimates were: 59% vs 40%, PFS; 59% vs 55%, OS; and 62% vs 66%, DOCR (supplemental Figure 3).
A total of 30 of 62 MRD-evaluable patients (48%) had MRD negativity at any time point (cycle 3 day 1 landmark analysis shown in supplemental Figure 4). By cycle 3 day 1, there was a decrease in circulating tumor DNA (median log10-fold decrease, 2.04), and MRD-negativity rates were 55% in patients with 1 pLOT and 49% in patients with ≥2 pLOT. Overall, MRD-negativity rates were 50% among patients with primary refractory disease and 47% among patients without primary refractory disease.
Safety
The most common TEAEs of any grade in the full analysis population were hematologic AEs, infections, and CRS; 73% of patients had thrombocytopenia, 65% had neutropenia, 59% had anemia, 72% had infections, and 52% had CRS. The most common grade ≥3 TEAEs were thrombocytopenia (59%), neutropenia (57%), and anemia (43%). Febrile neutropenia occurred in 7 patients (7%); all events were grade 3 and resolved. ICANS was reported in 3 patients (grades 1-3, n = 1 each). All ICANS events resolved, and 1 patient discontinued treatment because of ICANS. An overview of TEAEs is shown in Table 4.
Overview of TEAEs in the overall population
| Overall, N = 103 . | Any grade, n (%) . | Grade ≥3, n (%) . |
|---|---|---|
| Any AE | 103 (100.0) | 97 (94.2) |
| Serious AE | 76 (73.8) | 54 (52.4) |
| AE leading to treatment discontinuation | 36 (35.0) | 30 (29.1) |
| AEs in ≥15% of patients | ||
| Hematologic AEs | ||
| Thrombocytopenia∗ | 75 (72.8) | 61 (59.2) |
| Neutropenia† | 67 (65.0) | 59 (57.3) |
| Anemia‡ | 61 (59.2) | 44 (42.7) |
| Infections§ | 74 (71.8) | 30 (29.1) |
| COVID-19|| | 30 (29.1) | 11 (10.7) |
| CRS¶ | 54 (52.4) | 1 (1.0) |
| Diarrhea | 48 (46.6) | 5 (4.9) |
| Nausea | 41 (39.8) | 2 (1.9) |
| Fatigue | 36 (35.0) | 7 (6.8) |
| Hypokalemia | 32 (31.1) | 9 (8.7) |
| Pyrexia | 30 (29.1) | 0 |
| Increased alanine aminotransferase | 29 (28.2) | 10 (9.7) |
| Increased aspartate aminotransferase | 26 (25.2) | 6 (5.8) |
| Peripheral neuropathy | 26 (25.2) | 1 (1.0) |
| Cough | 21 (20.4) | 0 |
| Hypomagnesemia | 20 (19.4) | 0 |
| Decreased appetite | 19 (18.4) | 3 (2.9) |
| Increased lipase | 19 (18.4) | 6 (5.8) |
| Injection-site reaction# | 18 (17.5) | 0 |
| Dyspnea | 17 (16.5) | 2 (1.9) |
| Constipation | 16 (15.5) | 0 |
| AEs of special interest | ||
| CRS¶ | 54 (52.4) | 1 (1.0) |
| ICANS | 3 (2.9) | 1 (1.0) |
| Clinical tumor lysis syndrome | 0 | 0 |
| Overall, N = 103 . | Any grade, n (%) . | Grade ≥3, n (%) . |
|---|---|---|
| Any AE | 103 (100.0) | 97 (94.2) |
| Serious AE | 76 (73.8) | 54 (52.4) |
| AE leading to treatment discontinuation | 36 (35.0) | 30 (29.1) |
| AEs in ≥15% of patients | ||
| Hematologic AEs | ||
| Thrombocytopenia∗ | 75 (72.8) | 61 (59.2) |
| Neutropenia† | 67 (65.0) | 59 (57.3) |
| Anemia‡ | 61 (59.2) | 44 (42.7) |
| Infections§ | 74 (71.8) | 30 (29.1) |
| COVID-19|| | 30 (29.1) | 11 (10.7) |
| CRS¶ | 54 (52.4) | 1 (1.0) |
| Diarrhea | 48 (46.6) | 5 (4.9) |
| Nausea | 41 (39.8) | 2 (1.9) |
| Fatigue | 36 (35.0) | 7 (6.8) |
| Hypokalemia | 32 (31.1) | 9 (8.7) |
| Pyrexia | 30 (29.1) | 0 |
| Increased alanine aminotransferase | 29 (28.2) | 10 (9.7) |
| Increased aspartate aminotransferase | 26 (25.2) | 6 (5.8) |
| Peripheral neuropathy | 26 (25.2) | 1 (1.0) |
| Cough | 21 (20.4) | 0 |
| Hypomagnesemia | 20 (19.4) | 0 |
| Decreased appetite | 19 (18.4) | 3 (2.9) |
| Increased lipase | 19 (18.4) | 6 (5.8) |
| Injection-site reaction# | 18 (17.5) | 0 |
| Dyspnea | 17 (16.5) | 2 (1.9) |
| Constipation | 16 (15.5) | 0 |
| AEs of special interest | ||
| CRS¶ | 54 (52.4) | 1 (1.0) |
| ICANS | 3 (2.9) | 1 (1.0) |
| Clinical tumor lysis syndrome | 0 | 0 |
Thrombocytopenia includes hematopoietic thrombocytopenia using the Standardized Medical Dictionary for Regulatory Activities Query narrow search.
Neutropenia includes neutropenia and decreased neutrophil count.
Anemia includes anemia, decreased hematocrit, decreased hemoglobin, decreased red blood cell count, and decreased serum ferritin.
Infections and infestations by system organ class.
COVID-19 includes COVID-19, COVID-19 pneumonia, and post–acute COVID-19 syndrome.
CRS is shown twice (as an AE in ≥15% of patients and as an AE of special interest).
Injection-site reaction includes injection-site reactions as high-level group terms.
Overall, 13 patients (13%) had grade 5 TEAEs. Of these, 5 events were COVID-19. The study was largely enrolled during the COVID-19 pandemic. Further descriptions are provided in the supplemental Results.
CRS was primarily low grade (52% overall incidence; 28% grade 1, 23% grade 2); only 1 patient experienced grade 3 CRS. CRS events mostly occurred in cycle 1 (84% of CRS events), and more specifically, after the first full dose (63% of CRS events). All events resolved and none led to epcoritamab discontinuation. Tocilizumab was used in 24 patients (23%) and corticosteroids beyond those for prophylaxis were used in 17 (17%). An overview of CRS is shown in supplemental Table 3.
The rate of infections was 72%; most were COVID-19 (29%), upper respiratory tract infection (14%), pneumonia (10%), and urinary tract infection (10%). Overall, 32% of patients experienced serious infections, most of which were COVID-19 and pneumonia. Grade 3 or 4 infections were reported in 21% of patients. In the first 12 weeks of the trial, during which patients received epcoritamab plus GemOx, the incidence of infections was 56% and the incidence of grade 3 or 4 cytopenias was 76%; these rates decreased substantially in subsequent 12-week intervals up to week 60, to 22% to 28% for infections overall and 3% to 39% for grade 3 or 4 cytopenias. Four patients experienced secondary malignancies: 2 patients had basal cell carcinoma, 1 had pancreatic adenocarcinoma, and 1 had squamous cell carcinoma.
Immunoglobulin levels
Immunoglobulin levels decreased from baseline after treatment initiation and remained relatively stable up to cycle 18, with no larger mean decreases from baseline in immunoglobulin G levels over time (supplemental Figure 5). Overall, 22% of patients received supplemental immunoglobulin therapy.
Discussion
In EPCORE NHL-2 arm 5, epcoritamab plus GemOx treatment resulted in complete remissions in most patients with R/R DLBCL who were ineligible for ASCT or for whom ASCT had failed. ORR and CR rate were high at 85% and 61%, respectively. High CR rates were seen in patients with 1 pLOT (74%) and patients with no prior CAR T-cell therapy (68%). Responses were observed early and were deep and durable, translating to sustained CRs and favorable PFS; median OS was NR in patients with CR. Three patients with stable disease had a PR or CR after week 12, suggesting the potential for a later response. MRD negativity was observed in approximately half of evaluable patients; MRD-negativity rates by cycle 3 day 1 were slightly higher in patients with 1 pLOT than patients in later lines of therapy. Safety was in line with GemOx therapy and epcoritamab monotherapy and was generally manageable3,14,26; CRS events were low grade, comparable with single-agent epcoritamab data,27 and all resolved. These results demonstrate that the addition of epcoritamab to GemOx was feasible and follow several other studies assessing epcoritamab in combination with immunochemotherapy such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone); and R-DHAX/C (rituximab, dexamethasone, cytarabine, and oxaliplatin or carboplatin) in DLBCL, which collectively show the versatility and combinability of epcoritamab with chemotherapies.20,28
Responses to epcoritamab plus GemOx were similar to, or compared favorably with, those observed with other approved therapies, including historical R-GemOx data.3,26 However, cross-study comparisons should be interpreted with caution because of differences in patient populations and study designs; for example, patients in this study were treated with epcoritamab until disease progression, which is distinct from other therapies with a fixed duration. In this cohort of patients with advanced disease, 35% of patients were aged ≥75 years, 23% had transformed DLBCL, 61% had stage IV disease, 70% had disease refractory to their last systemic therapy, and 28% had prior CAR T-cell therapy. Enrolled patients were more likely to be in later lines of therapy (62% with ≥2 pLOT) than those with R/R DLBCL treated with R-GemOx (42% with ≥2 pLOT).3 In contrast to R-GemOx treatment, which yielded CR rates of ∼30% in patients with R/R DLBCL regardless of whether they had 1 or ≥2 pLOT,3 epcoritamab plus GemOx treatment led to higher CR rates overall (61%) and markedly improved CR rates in patients with 1 vs ≥2 pLOT (74% vs 53%), supporting that this combination may help to overcome cross-resistance. Median relative dose intensities of gemcitabine and oxaliplatin were similar to or higher compared with other studies, consistent with the nonoverlapping toxicities of epcoritamab and GemOx.3,26 Importantly, gemcitabine has been shown to enhance antitumor immune activity in part by suppressing regulatory T cells and, therefore, may be a contributor to the encouraging efficacy seen here.29,30
In a report of another bispecific antibody combination evaluated in the phase 3 STARGLO trial,4 efficacy among patients with ASCT-ineligible R/R DLBCL who received glofitamab plus GemOx every 3 weeks was comparable with that seen in EPCORE NHL-2 arm 5, including ORR and CR rate of 68% and 58% with median PFS and OS of 13.8 months and 25.5 months. However, EPCORE NHL-2 included a broader, harder-to-treat population, because several high-risk patient groups were excluded from STARGLO, including patients with double- or triple-hit lymphoma (EPCORE NHL-2 arm 5: n = 6; CR rate, 50%) and patients with recent COVID-19 (≤6 months of the first study dose). Additionally, EPCORE NHL-2 arm 5 included more patients with ≥2 pLOT (62% vs 37%) and prior CAR T-cell therapy (28% vs 7%) relative to STARGLO,4 and had minimal regional differences in OS. In another bispecific antibody combination approach, a phase 1b/2 trial evaluated mosunetuzumab with the antibody–drug conjugate polatuzumab vedotin for patients with R/R LBCL.31 The safety was manageable, with low rates of ICANS (5%) and grade 3 CRS (3%). Although most patients in the dose-expansion cohort did not have a CR, efficacy was promising, with ORR and CR rate of 59% and 46%, respectively, per IRC; however, treatment with epcoritamab plus GemOx has yielded higher response rates (85% and 61%). Notably, the mosunetuzumab study excluded patients with prior polatuzumab therapy, so it will be important to assess the efficacy of the approach given the recent approval of polatuzumab with first-line chemotherapy.32
The safety profile of epcoritamab plus GemOx was generally similar to that of other chemotherapy combinations in LBCL. High rates of neutropenia, including 73% of patients with grade ≥3 neutropenia, have been reported in patients with R/R DLBCL ineligible for high-dose therapy who received R-GemOx.26 In EPCORE NHL-2 arm 5, 57% of patients experienced grade ≥3 neutropenia (febrile neutropenia, 7%). CRS was primarily low grade (52% overall, 1% grade 3); rates were comparable with single-agent epcoritamab data.27 Three patients experienced ICANS (3% overall, 1% grade 3). Additionally, in this trial, 21% of patients experienced infections that were grade 3 or 4 at worst, which is comparable with historical R-GemOx studies (22% of cycles).26 Overall, 13% of patients experienced fatal TEAEs, most of which were COVID-19 or other infections. Of note, there is a well-documented increased risk of morbidity and mortality because of infections for patients with hematologic malignancies being treated with B-cell–depleting therapies, and this study overlapped with the COVID-19 pandemic.33,34 Furthermore, in this trial, patients received GemOx more frequently (every 2 weeks) than the less-frequent dosing strategy (every 3 weeks) used in the STARGLO study4; although less frequent administration may be less burdensome for patients, it may yield a greater risk of early kinetic failure, because dose density has been correlated with improved outcomes in lymphoma.35 It is promising that epcoritamab could be safely combined with a dose-dense chemotherapy regimen (GemOx) and that this combination therapy was sufficiently well tolerated.
Epcoritamab is a subcutaneous therapy that can offer off-the-shelf convenience compared with other available options. CAR T-cell therapies, such as axicabtagene ciloleucel, lisocabtagene maraleucel, and tisagenlecleucel, demonstrate long-term efficacy but may not be accessible to all patients and are not immediately available to patients with an urgent need for treatment due to requirements for lymphodepletion, access to specialized treatment centers, and manufacturing time.8,9,36-40 Of particular importance, the median age of patients in seminal CAR T-cell therapy trials was 56 to 60 years and trials lacked older patients (aged ≥75 years [highest age, 76 years]), compared with this study, in which median age was 72 years and more than one-third of patients were aged ≥75 years (highest age, 87 years), highlighting the relative accessibility and safety of this combination therapy.7,36,39 Notably, real-world analyses have demonstrated that CAR T-cell therapies have significantly shorter event-free survival in patients aged ≥75 years.41 The combination of epcoritamab and chemotherapy, such as GemOx, offers an effective treatment option to improve outcomes for these patients with an unmet need. As with other open-label, single-arm studies, EPCORE NHL-2 is limited by its lack of a comparator or control group. Additional limitations are a lack of racial and ethnic diversity in patients enrolled and missing race and ethnicity data because of regional guidelines. Longer follow-up is needed to better understand long-term outcomes.
In conclusion, results from EPCORE NHL-2 arm 5 demonstrated deep and durable responses with epcoritamab plus GemOx in a population of patients with ASCT-ineligible R/R DLBCL, most of whom had high-risk features. Epcoritamab plus GemOx treatment resulted in high response rates across subgroups, especially among patients with 1 pLOT. The addition of epcoritamab to GemOx demonstrated significantly improved outcomes compared with historical data for R-GemOx. These encouraging results support the combinability and versatility of epcoritamab in R/R DLBCL.
Acknowledgments
The authors thank the patients and their families for their participation in this study; the participating study sites, investigators, data monitoring committee, and other research personnel for their support of this trial; Phillip Chen for statistical analysis support; and David Soong for valuable contributions. Medical writing assistance was provided by Hannah L. Mayberry and Christina Mulvihill of Peloton Advantage, LLC, an OPEN Health company, and funded by Genmab A/S and AbbVie.
P.J.L. received research grants from Takeda and Servier.
Authorship
Contribution: R. Cordoba helped design the trial; J.D.B., J.J., D.B., R. Costello, M.T., U.V., D.J.L., Y.H.K., A.S., M.A., B.E.W., P.J.L., and R. Cordoba were study investigators and provided patients or study materials; J.D.B., J.J., D.B., R. Costello, M.T., U.V., D.J.L., Y.H.K., A.S., M.A., B.E.W., P.J.L., and R. Cordoba collected and assembled data; K.K., A.J.S., A.A., L.W., and M.R. analyzed the data; and all authors interpreted the data, prepared the manuscript, participated in the critical review and revision of the manuscript, and provided approval of the manuscript for submission.
Conflict-of-interest disclosure: J.D.B. reports advisory role for ADC Therapeutics, Epizyme, and Seagen; and research funding from Bristol Myers Squibb, Genentech, Gilead/Kite, and Merck. J.J. reports consultancy role for AbbVie, Gilead, Incyte, Orion, Roche, and Sobi. D.B. reports consultancy role for AbbVie, Bristol Myers Squibb, Genmab, Gilead, MorphoSys, Roche, Sobi, and Takeda; honoraria from AbbVie, Gilead, Janssen, MorphoSys, Roche, and Takeda; and travel accommodations/expenses from AbbVie, Recordati, and Roche. M.T. reports consultancy role for AbbVie, Amgen, Bristol Myers Squibb, Genmab, Gilead, Incyte, Janssen, MorphoSys, Novartis, Roche, Sobi, and Takeda; honoraria from AbbVie, Amgen, Bristol Myers Squibb, Gilead, Janssen, MorphoSys, Novartis, Roche, and Takeda; and travel accommodations/expenses from AbbVie, Bristol Myers Squibb, Gilead, Janssen, Roche, and Takeda. U.V. reports advisory role for AbbVie, Genmab, Gilead, Incyte, and Regeneron; and lecture fees from AbbVie, Gilead, Incyte, Janssen, Merck Sharp & Dohme, Regeneron, Roche, and Servier. D.J.L. reports advisory and consultancy roles for Janssen, Lilly, Roche, BeiGene, and Kite. Y.H.K. reports advisory or consultancy roles for AbbVie and ADC Therapeutics; travel expenses from Roche/Genentech; and research funding from AbbVie, AstraZeneca, Lilly/Loxo, Merck, Roche/Genentech, and Xencor. A.S. reports consultancy role for Takeda, Bristol Myers Squibb, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GlaxoSmithKline (GSK), Sanofi, Kite, and Mundipharma; honoraria from Takeda, Bristol Myers Squibb, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GSK, Sanofi, and Kite; membership on an entity’s board of directors or advisory role for Takeda, Bristol Myers Squibb, Novartis, Janssen, Amgen, Bluebird, Sanofi, and Kite; travel expenses from Takeda, Bristol Myers Squibb, and Roche; research funding from Takeda; and speaker’s role for Takeda, Bristol Myers Squibb, Novartis, Janssen, Merck Sharp & Dohme, Amgen, GSK, Sanofi, and Kite. B.E.W. reports consultancy role and research funding from Roche, and research funding from Gilead. P.J.L. reports advisory honoraria from Bristol Myers Squibb, Roche, Takeda, Genmab, AbbVie, Incyte, Regeneron, and Sandoz; and consultancy honoraria from Y-mAbs Therapeutics. T.J. reports current employment at AbbVie. K.K., A.J.S., A.A., L.W., and M.R. report current employment and stock ownership at Genmab. R. Cordoba reports consultancy role for AbbVie, Janssen, AstraZeneca, Kite, Bristol Myers Squibb, Genmab, Roche, Takeda, Kyowa Kirin, BeiGene, and Lilly; speaker’s role for AbbVie, Janssen, AstraZeneca, Kite, Bristol Myers Squibb, Roche, and Takeda; and research funding from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Joshua D. Brody, Icahn School of Medicine, 1 Gustave L. Levy Pl, New York, NY 10029; email: joshua.brody@mssm.edu.
References
Author notes
Documents such as the study protocol, statistical analysis plan, informed consent form, and others will be made available on request for further analyses by external independent researchers. However, deidentified individual participant data collected during the trial will not be available. Aggregated clinical trial data from the trial are provided via publicly accessible study registries/databases as required by law. For more information, please contact clinicaltrials@genmab.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal