In this issue of Blood, Conrad et al report that the adult human lung hosts a population of hematopoietic stem and progenitor cells (HSPCs) comparable to that of bone marrow (BM) in quantity, but with a distinct transcriptomic profile.1 Although the presence of lung HSPCs has been described previously, the current work provides a new perspective on the potential influence of human lung HSPCs both locally within the lung and after mobilization.
Throughout development, hematopoiesis occurs in many different stages and locations, and it has a significant impact on the ultimate function of differentiated blood cells.2 There has been increasing interest in the lung as a previously underappreciated site of hematopoiesis. Lung megakaryocytes (MKs) are skewed toward functions in immunity and are capable of significant platelet production under both steady-state and thrombocytopenic conditions.3-5 Adult and fetal lung HSPCs and hemogenic endothelium have also been described.3,6 However, little is known about whether lung HSPCs directly contribute to hematopoietic populations found locally in the lung.
A recent publication demonstrated that murine lung MKs are derived mainly from BM hematopoietic stem cells (HSCs) through an Flt3-independent, direct differentiation pathway, but may also be generated from proliferation of lung MKs and/or differentiation from in situ lung HSPCs.7 Using single-cell RNA sequencing on donor human lung–derived samples, Conrad et al show that lung HSPCs are enriched for genes related to platelet function and immune signaling, suggesting a potential shift toward megakaryopoiesis, similar to previously described MK-biased HSCs8 that may contribute directly to the lung MK pool. The need for such a bias is not known, but a possible reason may stem from the functional role of MKs produced from biased HSCs.
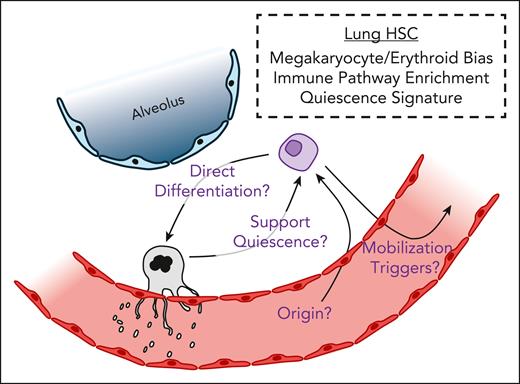
Others have demonstrated that MK-biased HSCs that undergo a more direct differentiation route tend to produce niche-supporting MKs that remain closer to HSCs and promote HSC quiescence.9 Although the majority of lung MKs have an immune profile, a smaller fraction of murine lung MKs are niche supporting.10 Conrad et al found that lung HSCs are enriched for genes related to quiescence. The fate of lung HSCs remains to be determined, but one possibility is that they may be producing MKs specifically to support their maintenance in this specialized niche. Further work is needed to identify this potential mechanism and others that contribute to lung HSC maintenance (see figure).
The finding that lung HSCs prioritize quiescence is of interest when considering their microenvironment. Using spatial transcriptomics on donor human lung samples, the authors have shown that the majority of lung HSCs are in the alveolar interstitium of the distal lung where blood and gas exchange occur. As such, lung HSCs are likely under considerable oxidative and inflammatory stress secondary to the various inhalation exposures that the lung epithelium endures, including smoking, air pollutants, and various microbes. Because stress is a significant stimulus for HSC differentiation and exhaustion,11 lung HSCs may prioritize maintaining quiescence to attenuate stress-induced hematopoiesis. However, understanding their triggers to differentiate are also of importance.
Studies in mice have shown that, under thrombocytopenic conditions, there is an increase in lung MKs that are derived through an Flt3-dependent, stepwise differentiation.7 This may be reflective of a shift in local lung HSCs shifting away from quiescence in an effort to support platelet reconstitution. Despite their potential MK bias, lung HSCs are also capable of engraftment and multilineage differentiation, as demonstrated in xenotransplantation studies. The authors also show that, in healthy volunteers given granulocyte colony-stimulating factor, approximately 15% of mobilized HSCs had a gene expression pattern indicating that they may have been derived from the lung. As such, lung HSCs may serve as a significant reservoir of HSCs capable of hematopoietic reconstitution. It remains unknown when, or if, lung HSCs are mobilized under physiologic conditions (see figure).
Overall, Conrad et al present significant implications that broaden our understanding of the complex developmental interplay between the lung, BM, and other sites of hematopoiesis from embryogenesis through adulthood. Despite this, our understanding of the lung’s hematopoietic potential remains in its infancy. In addition to understanding mechanistically how lung HSCs are influenced by their niche, the origin and fate of lung HSCs remain unknown (see figure). Investigating such questions would require the adaptation or development of technologies that allow for lung-specific HSC lineage tracing.
Defining the signature and impact of lung HSCs. Lung HSCs have a bias toward megakaryopoiesis and erythropoiesis. They are also enriched for genes related to immune pathways and the maintenance of quiescence. The overwhelming majority of lung HSCs reside within the alveolar interstitium where gas exchange between inhaled air and the dense pulmonary capillary networks takes place. Several questions remain, highlighted in purple text, regarding the physiologic impact of lung HSCs. Do lung HSCs contribute directly to the lung megakaryocyte pool and in return support local HSC quiescence? What are the origins of lung HSCs? How and when are lung HSCs mobilized?
Defining the signature and impact of lung HSCs. Lung HSCs have a bias toward megakaryopoiesis and erythropoiesis. They are also enriched for genes related to immune pathways and the maintenance of quiescence. The overwhelming majority of lung HSCs reside within the alveolar interstitium where gas exchange between inhaled air and the dense pulmonary capillary networks takes place. Several questions remain, highlighted in purple text, regarding the physiologic impact of lung HSCs. Do lung HSCs contribute directly to the lung megakaryocyte pool and in return support local HSC quiescence? What are the origins of lung HSCs? How and when are lung HSCs mobilized?
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal