In this issue of Blood, Mansoori Moghadam et al have made an important step forward in understanding the colitis associated with chronic granulomatous disorder (CGD)1; the investigators have strongly implicated the microbiota as an essential contributor to this disease and identified mononuclear phagocytes as the key host cell lineage involved.
CGD is an inborn error of immunity caused by mutations in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase NOX2, leading to impaired phagocyte oxidative burst. In addition to life-threatening infections, affected patients suffer inflammatory complications including a distinct form of colitis, histologically similar to Crohn’s disease but with a distribution usually mirroring ulcerative colitis.2 The condition confers significant morbidity and commonly requires major surgical intervention.3 However, its pathogenesis remains poorly understood while treatments, for example, with therapeutic monoclonal antibodies, are variably effective and may be complicated by severe infection.4,5 The only widely accepted pharmacological intervention is corticosteroids, and the only known curative intervention is stem cell transplant.
Historically, one issue with trying to understand CGD-associated colitis has been a lack of preclinical models, with CGD mice not accurately recapitulating the human disease. Indeed, the authors confirm some of the problems here, including a lack of spontaneous colitis in affected mice (albeit with a greater susceptibility to chemically induced colitis), greater severity in p47phox knockout animals vs gp91phox knockout (when the pattern is the opposite in humans), and only partial rescue of the susceptibility phenotype with stem cell transplant. However, they do demonstrate some differences vs wild-type mice, especially following induced colitis, and these point toward monocytes and macrophages as key mediators of disease. Interestingly, pathological changes are noted in this cell lineage in the bone marrow (including presumed “emergency monocytopoiesis”) as well as at the disease site.
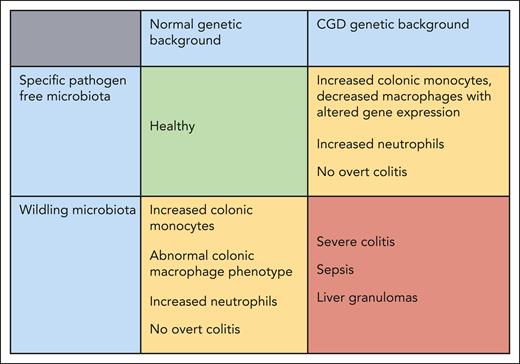
The real strength of this work was in the study of the microbiota. In trying to understand why the phenotype was paradoxically milder in gp91phox knockout mice, the authors noted important differences in the microbiota between the various groups of animals. As per standard laboratory practice, the mice had been kept in specific pathogen-free conditions. By cross-fostering CGD pups to introduce a “wildling” microbiota, the investigators were able to recapitulate a severe phenotype with the development of spontaneous colitis, as well as granuloma formation in the liver, and no difference between the 2 CGD genetic backgrounds (see figure). Notably, there were changes in gut-associated phagocyte populations even in wild-type mice exposed to the wildling microbiota.
Summary of the key findings as reported in the paper by Mansoori Moghadam et al. The CGD genetic background in combination with a specific pathogen-free (standard laboratory) microbiota or a healthy genetic background with a wildling microbiota both lead to abnormalities of colonic phagocytes and low-grade inflammation but no overt colitis. However, the combination of the CGD genetic background and wildling microbiota leads to severe spontaneous colitis with translocation of bacterial species and distant granuloma formation.
Summary of the key findings as reported in the paper by Mansoori Moghadam et al. The CGD genetic background in combination with a specific pathogen-free (standard laboratory) microbiota or a healthy genetic background with a wildling microbiota both lead to abnormalities of colonic phagocytes and low-grade inflammation but no overt colitis. However, the combination of the CGD genetic background and wildling microbiota leads to severe spontaneous colitis with translocation of bacterial species and distant granuloma formation.
Although pathological alterations in microbiome are described in human CGD,6,7 and in inflammatory bowel disease more generally,8 these data tend to be observational, and it is difficult to disentangle cause from effect. It is inevitable that the microbiota associating with inflamed bowel tissue in a patient exposed to multiple treatments (both antimicrobial and immunosuppressive) will be different from healthy controls. But here, the authors directly implicate an essential role for the microbiota in the development of colitis, a significant contribution to understanding disease pathogenesis.
The model should now allow dissection of pathological mechanisms, both host and microbial. The authors proceeded to further implicate mononuclear phagocytes: mice with impaired NOX2 function only in macrophages (and intact in neutrophils) developed a similar phenotype on exposure to the wildling microbiota. However, these cells could be further investigated, including their cytokine and chemokine profile, especially focusing on those molecules with clinically available therapeutic monoclonal antibodies. Interesting data already exist implicating NADPH oxidase in antigen presentation by professional antigen-presenting cells via both major histocompatibility complex I (MHC-I) and MHC-II,9,10 and this could be looked at further, perhaps on exposure to both commensal and pathological bacteria. Is there any role at all for granulocytes, including a “bystander effect,” that is the impact of neutrophils undergoing aberrant cell death on macrophage gene expression, antigen presentation, and cytokine release?
The authors implicate certain bacterial taxa in the colitis phenotype, but this can also be investigated further, in particular the metabolic function of those genera, which appear to confer either risk or protection.7 Such an analysis may be of more relevance for extrapolation to humans: the species present in mouse and human guts are likely to differ, but microbial function may be more similar.
The model should also provide the opportunity to study clinically important questions. For example, what is the role of antibiotic and antifungal prophylaxis, given universally to patients with CGD, on the microbiota and subsequent development of colitis? What is the response to therapeutic interventions for colitis, including clinically available monoclonal antibodies, or to deliberate modifications of the microbiome (eg, via fecal transplant)? How do curative interventions for CGD, including novel techniques such as gene editing, impact the colitis phenotype?
The introduction of wildling microbiota, more accurately replicating “real-world” settings, could also be studied in other inborn errors of immunity conferring a risk of colitis, and, in turn, these insights are likely to be useful to understand all forms of inflammatory bowel disease.
There are also intriguing data in this article suggesting the importance of the gut-bone marrow axis. The microbiota is increasingly implicated in disease distant to the gut, and we have previously demonstrated differences in mucosal microbiome according to the level of systemic inflammation in CGD, regardless of cause.7 Again, this demands more study.
Of course, any findings in the mouse model will need to be confirmed in human studies, and one limitation of the current article was the small number of human samples available. Other potential issues included the exact timing of cross-fostering and the study of neutrophils being limited to numbers only.
Nevertheless, this work has overcome major issues with mouse models of CGD colitis, has directly implicated the microbiota in the development of this condition (previously only assumed but never proven), and has highlighted mononuclear phagocytes rather than neutrophils as the key players in pathogenesis. This is a solid foundation for translational work to improve outcomes for patients.
Conflict-of-interest disclosure: D.M.L. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal