In this issue of Blood, Santocki et al1 uncover the presence of neutrophil extracellular trap (NET) proteins adhered to the endothelial wall for an extended period following endotoxemia. These NET remnants were further shown to induce the formation of a subsequent NET wave(s).
Neutrophils represent the overwhelming majority of circulating leukocytes.2 It is therefore not surprising that a relationship exists between neutrophil activity and vascular homeostasis.3,4 Neutrophils are prominent mediators of both microbial clearance and wound healing.2 One of their signature functions is the production of NETs, referred to as NETosis.3 During NETosis, neutrophils disassemble their nucleus and release DNA, histones, and cytotoxic proteins, requiring neutrophil elastase (NE) and peptidyl arginine deiminase type IV.5 NETs ensnare microbes and microbe structures, leading to pathogen eradication.3 However, this last gasp effort to eradicate bloodstream infections comes at a heavy cost, injuring endothelium and other host cells.5 This is especially problematic in the vasculature, where a delicate balance between pro- and anticoagulation factors exists and NETs can induce thrombin activity and coagulation.4 Not surprisingly, NET formation in the bloodstream is often associated with procoagulatory activity, and as such NETs have been linked to the pathogenesis of hematologic diseases.5 This has prompted research into NET neutralization with much emphasis on DNAses, enzymes that degrade DNA.5 However, both endogenous and therapeutic DNAses, which rapidly degrade DNA, should theoretically have no inhibitory effect on other toxic molecules associated with NETs, such as histones and elastase.
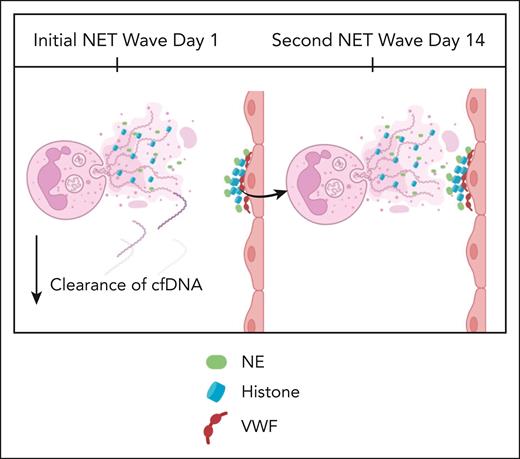
The article by Santocki et al investigated the process of NET clearance using elegant in vivo intravital microscopy imaging. They used lipopolysaccharide (LPS) to show that NET-associated DNA was cleared quickly, but histones and NET proteins that had adhered to the endothelial wall persisted for 160 and up to 365 days, respectively (see figure). Previous research in vivo has demonstrated that histones can bind avidly to von Willebrand factor exposed on endothelium,5 potentially securing NETs to the wall of the vasculature. DNAse removed DNA, but the remaining NET remnants were able to induce an unexpected second wave of NET formation at around 14 days. Whether this cycle continues for months is not clear from this study but does raise the interesting possibility that NETs, once formed, can have ongoing long-term effects. This recurrent process could be important in a number of conditions including sepsis and COVID-19 infections as well as chronic conditions such as diabetes, atherosclerosis, and autoimmunity.5
Santocki et al demonstrated that following initial NETosis response, exposed NET remnant histones induce a secondary wave of NETosis. cfDNA, circulating free DNA; VWF, von Willebrand factor. Figure created with BioRender.com.
Santocki et al demonstrated that following initial NETosis response, exposed NET remnant histones induce a secondary wave of NETosis. cfDNA, circulating free DNA; VWF, von Willebrand factor. Figure created with BioRender.com.
With respect to sepsis, there is a little recognized condition termed “postsepsis syndrome,” “post-ICU (intensive care unit) syndrome,” or “long sepsis,”6 and public awareness only occurred after the wave of survivors from COVID-19 (sepsis caused by SARS-CoV-2 infection) yielded tens of thousands now suffering from “long COVID/long sepsis.”7 This condition is marked by an increased threat of death upon discharge. No less than 40% of patients are readmitted to hospital with repeated episodes of sepsislike syndrome, and more patients die in the first year postsepsis than from the initial septic episode.8,9 It would seem reasonable to consider the recurrent NET production as a possible underlying cause of long sepsis, highlighting the potential importance of this study.
NE and histones (NET remnants) proved difficult for the body to remove from the endothelial wall naturally and required opsonization by antibodies, which Santocki et al showed were then taken up by the liver macrophages or Kupffer cells that reside in the hepatic microcirculation as well as neutrophils. Whether these were newly made antibodies in response to the extracellular elastase and histones or whether these were natural antibodies remained unexplored in this study. Moreover, whether the 14-day renewed surge of NETs was related to antibody production was also not clear. Nevertheless, this discovery begs the question of whether therapeutic antibodies or vaccines could be made to help remove NETs in a more timely and effective manner.
A new concept of trained immunity has emerged that suggests that innate immune cells could have memory.10 This theory postulates that neutrophil “memory” exists due to epigenetic changes in neutrophil stem cell progenitors.10 Along these lines, the response to a bacterial infection given 165 days after the initial LPS stimulus caused a greatly enhanced response. This suggests that either the neutrophils have been epigenetically altered (trained) or the lingering NET remnants could somehow enhance the bacterial response. Elucidating the mechanisms underlying these observations is an obvious new direction.
In conclusion, this study unveils some interesting new biology including the recurrent production of NETs weeks after an initial insult that could explain ongoing pathology in some human diseases. Whether this is a one-time event a few weeks after the initial insult or multiple recurrences remains to be elucidated. Regardless, this study further stokes the need for the development of therapies that can either enhance NET removal or prevent NETosis altogether.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal