In this issue of Blood, Kater et al report on the final data cutoff of the MURANO trial, which compared 2-year fixed-duration venetoclax-rituximab (Ven-R) to 6 cycles of bendamustine-rituximab (BR) in patients with relapsed/refractory chronic lymphocytic leukemia (CLL).1
Over 10 years ago, this trial was initiated as the first randomized clinical trial with the BCL2 inhibitor Ven.2 Since then, Ven has become a cornerstone of the treatment of CLL. Previous reports have shown the high efficacy of Ven-R in relapsed/refractory CLL. The latest analyses with an impressive median observation time of 84 months indicate a 7-year overall survival (OS) rate of 70% and a 7-year progression-free survival (PFS) rate of 23%. Notably, in the largest prospective retreatment study so far, 25 patients who relapsed after Ven-R were retreated with Ven-R and had overall response rates of 72% and a median PFS of 23 months.
So, what have we learned from 7 years of MURANO? First, it must be acknowledged that to get meaningful data on the long-term implications of novel therapies in CLL, long-term observations like this are critical, since the disease dynamics and measurable disease (MRD) rates can only be properly understood when patients are followed regularly beyond the study primary end point. As seen now in MURANO, the majority of patients eventually experience relapses, as only one-fifth of the patients remained without a PFS event after 7 years. This highlights that there is still a need to improve the efficacy of the Ven-R regimen to increase the rate of long-term responders. To explore the causes of relapse, the authors examined the clonal composition of relapsed disease following fixed-duration Ven. Unlike previous studies on continuous Ven, which showed treatment-associated selection of BCL2 variants,3 acquired BCL2 mutations were initially undetected in MURANO. However, using sensitive droplet digital polymerase chain reaction with a limit of detection at 0.1%, 3-point mutations, including the common G101V variant, were now observed in 4 patients in the Ven-R arm. In contrast to frontline 1-year Ven-obinutuzumab, where BCL2 mutations so far have not been detected,4 the longer exposure to Ven over 2 years might have posed a sufficient selective pressure to facilitate the evolution of such variants in MURANO. The clinical relevance of these variants warrants further investigation, as they did not significantly prevent response to Ven retreatment. In the MURANO substudy, where patients with progressive disease received Ven-R as retreatment or crossover from BR, the data are encouraging, with response rates exceeding 70%, though undetectable MRD (uMRD) rates remained low, and the median PFS was under 2 years. This suggests that interval therapy (that is, administration of a limited number of treatment cycles, several years off therapy, and then retreatment with another course of fixed treatment cycles) can generate long-term disease control, as seen by the encouraging 7-year OS rate of 70% (see figure). Ongoing studies in earlier lines, such as the ReVenG trial that tests retreatment with fixed-duration Ven-obinutuzumab as a second-line treatment, will further shed light on the potential of retreatment and interval therapy (NCT04895436).
The MURANO trial produced critical evidence on the role of MRD in CLL. The investigators expended considerable effort to capture MRD longitudinally to reconstruct MRD dynamics; the final analyses reveal not only the strong prognostic impact of MRD on PFS, but also on OS. In addition, the longitudinal MRD assessments indicated only limited contribution of the last months of Ven in the 2-year schedule to the deepening MRD levels.5 Several strategies could improve outcomes in relapsed/refractory CLL. One option is to increase uMRD rates, which were 50% to 60% at the end of treatment in MURANO, by incorporating additional combination partners like BTK inhibitors. In frontline treatment, BTK inhibitors have increased uMRD rates from 57% with Ven-R to 92% with ibrutinib-Ven-obinutuzumab.6 Naturally, the additional toxicity linked with triple therapy requires careful clinical risk-benefit assessment. The ongoing BRUIN CLL-322 trial explores the triple combination of pirtobrutinib-Ven-R to Ven-R (ie, the MURANO regimen) in relapsed/refractory disease (NCT04965493). Moreover, modifying the combination partners, for example, by the use of a type 2 CD20 antibody like obinutuzumab, which is associated with substantially higher rates of uMRD than R in CLL11 and in CLL13,6,7 with a significant gain in PFS (and even OS in CLL11), could also have a benefit in the relapsed/refractory setting of CLL. Hence, the efforts made to generate data on R vs obinutuzumab in the frontline setting should be mirrored in the relapsed/refractory CLL setting. In addition to the CD20 antibody, exploration of next-generation Bcl-2 inhibitors, such as sonrotoclax or lisaftoclax, also requires prospective head-to-head evaluation against the Ven-R regimen to identify the added clinical benefit of these next-generation compounds. Finally, given the multiple lessons from the MRD dynamics analyses in MURANO, the question on treatment duration should be revisited in the frontline setting, including the data from the first randomized data on MRD guidance, such as the FLAIR trial,8 but also the ongoing CLL18, MAJIC (NCT05057494), or RESOLVE trials, which are exploring treatment shortening or extension based on MRD assessments. Such efforts have so far not been explored in relapsed CLL. The more or less arbitrary nature of the 2-year treatment schedule in MURANO, as well as the uncertain added value of the final months of Ven monotherapy in patients with relapsed/refractory CLL, provides a strong rationale to also systematically explore MRD-guided strategies in relapsed/refractory CLL.
In conclusion, the final readout of the MURANO study confirms the value of fixed-duration BCL2 inhibition in relapsed/refractory CLL and provides promising evidence for retreatment and interval therapy of CLL. Exploring MRD-guided strategies and alternative combination partners is a key next step in advancing therapy of relapsed/refractory CLL.
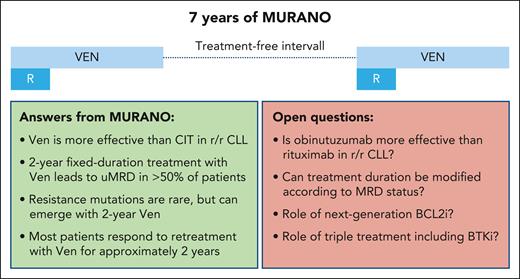
Answered and open questions from 7 years of the MURANO trial. CIT, chemoimmunotherapy; BCL2i, BCL2 inhibitor; BTKi, BTK inhibitor; r/r, relapsed/refractory.
Answered and open questions from 7 years of the MURANO trial. CIT, chemoimmunotherapy; BCL2i, BCL2 inhibitor; BTKi, BTK inhibitor; r/r, relapsed/refractory.
Conflict-of-interest disclosure: O.A.-S. is a member of the advisory boards of Ascentage, AstraZeneca, AbbVie, Genmab, Gilead, Janssen, and Roche; receives speaker honoraria from Adaptive, AstraZeneca, AbbVie, BeiGene, Gilead, Janssen, and Roche; and receives research funding from BeiGene, AbbVie, Janssen, and Roche.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal