Key Points
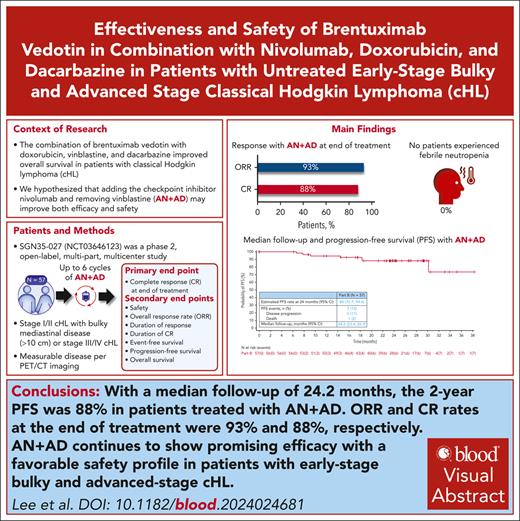
With a median follow-up of 24.2 months, the 2-year progression-free survival of patients treated with AN+AD was 88%.
AN+AD led to an 88% CR rate and favorable safety profile, notable for the absence of febrile neutropenia.
Visual Abstract
Treatment options for stage I/II bulky and advanced-stage disease have recently extensively changed. For decades in North America, ABVD (doxorubicin hydrochloride [Adriamycin], bleomycin sulfate, vinblastine sulfate, and dacarbazine) has been a frontline standard-of-care option for patients with advanced classical Hodgkin lymphoma (cHL). Recent data on combining brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine demonstrated improved overall survival compared with ABVD but increased adverse events (AEs). We hypothesized that replacing vinblastine with nivolumab (brentuximab vedotin and nivolumab [AN] + doxorubicin and dacarbazine [AD]; AN+AD) may improve efficacy and safety. This phase 2, open-label multipart, multicenter study enrolled patients with treatment-naive stage II bulky or III/IV cHL. Patients received ≤6 cycles of AN+AD; granulocyte-colony stimulating factor (G-CSF) prophylaxis was optional, per institutional guidelines. At the time of planned analysis (N = 57), complete response (CR) and objective response rates were 88% (95% confidence interval [CI], 76.3-94.9) and 93% (95% CI, 83.0-98.1), respectively. With a median follow-up of 24.2 months (95% CI, 23.4-26.9), the 2-year progression-free survival rate was 88% (95% CI, 75.7-94.6); 88% (95% CI, 75.7-94.6) had a response lasting >2 years. Most common grade ≥3 treatment-related AEs were alanine aminotransferase increased (11%) and neutropenia (9%); 44% had treatment-related peripheral sensory neuropathy (grade 1/2, 40%; grade 3, 4%). No febrile neutropenia occurred; 49% received G-CSF prophylaxis. AN+AD led to a high CR rate and favorable safety profile. Further evaluation of programmed death receptor 1 inhibitor and CD30 antibody–drug conjugate combination regimens in frontline advanced-stage cHL is warranted. This trial was registered at www.clinicaltrials.gov as #NCT03646123 and www.clinicaltrialsregister.eu as #EudraCT 2020-004027-17.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 348.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning objectives
Upon completion of this activity, participants will:
Describe efficacy of brentuximab vedotin, nivolumab, doxorubicin, and dacarbazine (AN+AD) treatment in patients with treatment-naive stage II bulky or III/IV classical Hodgkin lymphoma (cHL), based on planned analysis of data from 57 patients in a phase 2, open-label multipart, multicenter study
Determine the safety of AN+AD treatment in patients with treatment-naive stage II bulky or III/IV cHL, based on planned analysis of data from 57 patients in a phase 2, open-label multipart, multicenter study
Identify clinical implications of efficacy and safety of AN+AD treatment in patients with treatment-naive stage II bulky or III/IV cHL, based on planned analysis of data from 57 patients in a phase 2, open-label multipart, multicenter study
Release date: January 16, 2025; Expiration date: January 16, 2026
Introduction
In 2022, an estimated 8540 new cases of classical Hodgkin lymphoma (cHL) were diagnosed in the United States,1 with ∼40% of these patients diagnosed with advanced cHL.2,3 Although outcomes for patients with advanced cHL have improved, historical treatment options (eg, doxorubicin hydrochloride [Adriamycin], bleomycin sulfate, vinblastine sulfate, and dacarbazine [ABVD]) are associated with serious toxicities.4-10
Brentuximab vedotin is an antibody-drug conjugate composed of an anti-CD30 monoclonal antibody conjugated by a protease-cleavable linker to the microtubule-disrupting agent, monomethyl auristatin E, allowing for targeted delivery of the monomethyl auristatin E payload into CD30-expressing cells. In addition to direct cytotoxicity, some molecular studies have proposed other mechanisms of action (MOA), leading to cellular disruption.11-14
Based on the phase 3 ECHELON-1 trial, brentuximab vedotin in combination with AVD (doxorubicin, vinblastine, and dacarbazine; A+AVD) is approved as a frontline standard-of-care (SOC) option for patients with advanced cHL. A+AVD showed improved overall survival (OS; 6-year rates, 94% vs 89%) compared with ABVD, resulting in a 41% reduction in death.15 Improvement in OS with A+AVD was observed, despite use of salvage therapies in patients with relapsed/refractory disease. Furthermore, A+AVD has shown durable responses; 6-year progression-free survival (PFS) estimates were 82% vs 75% with A+AVD or ABVD, respectively.15 Other treatment options for stage III-IV cHL, based on recent trial results, include BrECADD (brentuximab vedotin, etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone) or nivolumab plus AVD (N+AVD).16
Despite improved efficacy with A+AVD, rates of peripheral neuropathy (PN) and febrile neutropenia were higher with this regimen, leading to inclusion of primary granulocyte-colony stimulating factor (G-CSF) prophylaxis as an amendment to the study.8,15 Toxicity associated with A+AVD may result from the overlapping MOA of brentuximab vedotin and vinblastine, which are both microtubule disrupters, and are associated with neuropathy and myelosuppression. In a phase 2 study of A+AD, omitting vinblastine, bleomycin, and radiation therapy, comparable response rates to A+AVD were observed in nonbulky limited-stage cHL, with an improved safety profile, warranting further investigation of brentuximab vedotin in combination with complementary and nonoverlapping agents in early and advanced disease.10
Nivolumab is a fully humanized monoclonal antibody that targets programmed death receptor 1 (PD-1). Nivolumab is approved as a monotherapy for relapsed/refractory cHL; high response rates and a tolerable safety profile are observed in combination regimens, including those with brentuximab vedotin.17-22 PD-1 blockade in combination with brentuximab vedotin may increase circulating T-cell subsets, inflammatory cytokines and chemokines, and the ability of memory T cells to mount an immune response, enhancing overall immune response to malignancies.13 Based on the demonstrated efficacy of brentuximab vedotin–based regimens,10,15 and distinct, yet potentially complimentary MOAs of brentuximab vedotin and nivolumab (AN), we hypothesized that combining AN with doxorubicin and dacarbazine (AN+AD) would result in high response rates and a well-tolerated safety profile, with potentially less toxicity than vinblastine-containing regimens in early and advanced disease.
Both brentuximab vedotin- and nivolumab-based therapies have changed frontline treatment of advanced cHL. For instance, A+AVD is approved for frontline treatment of advanced cHL, based on results of ECHELON-1.15 The phase 3 SWOG S1826 study in newly diagnosed, advanced (stage III/IV) cHL may be setting a new standard therapy. In this study, N+AVD demonstrated short-term, interim superiority compared with SOC A+AVD (1-year PFS rates, 94% vs 86%, respectively).23 Other treatment regimens have also been well-established in this setting, including BrECADD. The phase 3 German Hodgkin Study group (GHSG) HD21 trial of BrECADD vs eBEACOPP (escalated doses of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), each followed by radiotherapy in patients with residual positron emission tomography (PET)–positive disease showed PFS superiority of BrECADD to eBEACOPP (4-year rates, 94% vs 91%, respectively) and improved safety.24 In the phase 3 GHSG HD18 trial, in patients with PET-negative disease (Deauville score of 1-2), 5-year PFS rates were 91.2% and 93.0% in patients given 6 to 8 or 4 cycles of eBEACOPP, respectively.25
In North America, A+AVD (from ECHELON-1) is routinely used in the community setting. In addition, the PET-adapted approach after 2 cycles of ABVD used in the phase 3 RATHL study is also commonly used. Patients with PET-negative disease (Deauville score of 1-3) given AVD and those with PET-positive disease (Deauville score of 4-5) given BEACOPP had 3-year PFS rates of 84.4% vs 67.5%, respectively.26 Given the newly released data from the ECHELON-1, RATHL, GHSG HD21, and SWOG S1826 trials, the treatment landscape of advanced cHL will continue to evolve.
Here, we present efficacy and safety results from SGN35-027 (ClinicalTrials.gov identifier: NCT03646123; EudraCT number 2020-004027-17), a phase 2, open-label, multipart, multicenter, clinical trial for treatment-naive patients with bulky stage I/II and III/IV cHL treated with up to 6 cycles of AN+AD.
Patients and methods
Trial participants
Eligible patients were aged ≥12 years with treatment-naive histologically confirmed Ann Arbor stage I or II cHL with bulky mediastinal disease (≥10 cm), or stage III or IV cHL. Patients had bidimensional measurable disease, as documented by PET/computed tomography (CT) or CT imaging and at least 1 lesion of >1.5 cm in the longest diameter on cross-sectional imaging, measurable in 2 perpendicular dimensions on CT (or magnetic resonance imaging) and fluorodeoxyglucose by avid PET. Patients had an Eastern Cooperative Oncology Group performance status of 0, 1, or 2. Patients were excluded if they had any of the following: nodular lymphocyte predominant HL, active cerebral/meningeal disease related to underlying malignancy, including signs or symptoms of progressive multifocal leukoencephalopathy, prior immunosuppressive chemotherapy, therapeutic radiation, or any immunotherapy within 4 weeks of the first study drug dose or prior treatment with an anti–PD-1, anti–programmed death-ligand 1, anti–programmed death-ligand 2, or anti–cytotoxic T-lymphocyte associated protein 4 antibody. Full eligibility criteria are provided in the study protocol (supplemental Material, available on the Blood website).
Study design and treatment
Patients received up to 6 cycles of AN+AD (brentuximab vedotin 1.2 mg/kg [A], capped at a maximum body weight of 100 kg, nivolumab 240 mg [N], doxorubicin 25 mg/m2 [A], and dacarbazine 375 mg/m2 [D]). All study drugs were administered separately by intravenous infusions on days 1 and 15 of each 28-day cycle. Study drugs were administered in the following order: doxorubicin and dacarbazine, followed by brentuximab vedotin, followed by nivolumab. Nivolumab was administered ≥30 minutes after the end of the brentuximab vedotin infusion. G-CSF prophylaxis was not required per protocol and was administered at investigator’s discretion. Prophylactic medication use, including corticosteroids (<10 mg daily prednisone or equivalent, per local SOC), for nausea and vomiting was allowed.
Trial oversight
This study was designed by the sponsors in collaboration with an advisory committee. The trial received approval from site independent institutional review boards or ethics committees and was conducted in accordance with the Declaration of Helsinki and with good clinical practice guidelines defined by the International Council for Harmonisation. All patients provided written informed consent. The trial was sponsored by Seagen Inc (Bothell, Washington), which was acquired by Pfizer, Inc in December 2023. The authors vouch for the accuracy and completeness of the data and for adherence to the protocol. A safety monitoring committee composed of the study medical monitor, study biostatistician, drug safety representative, and study site investigators reviewed preliminary safety and efficacy data after∼10 patients completed cycle-2 dosing and approved continued enrollment. This manuscript reports results of a planned analysis.
End points
The primary end point was the complete response (CR) rate at the end of treatment (EOT). Secondary end points include assessments of safety and tolerability, overall response rate (ORR), duration of response (DOR), duration of CR (DOCR), event-free survival, PFS, and OS.
Assessments
Staging was performed using CT scans of diagnostic quality and PET scans at baseline, during days 25 to 28 of cycle 2, at EOT, and during long-term follow-up. Disease involvement was determined by focal fluorodeoxyglucose uptake in nodal and visceral sites that was consistent with lymphoma. PET scan metabolic uptake was graded using the Deauville 5-point scale with a score of ≤3, which was considered a complete metabolic response.27 Patients in long-term follow-up did not require PET if prior PET was negative; instead, imaging assessments were done by CT. All imaging assessments were performed by investigators. Safety evaluations included of the surveillance and recording of adverse events (AEs) including serious AEs, concomitant medications, physical examinations, and laboratory test results. Investigator-assessed AEs were graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Disease response and progression were assessed by investigator using Lugano Classification Revised Staging System for malignant lymphoma,28 incorporating Lymphoma Response to Immunomodulatory Therapy Criteria for nodal non-Hodgkin and Hodgkin lymphomas.29
Statistical analysis
A sample size of 50 was targeted for enrollment to provide an adequate level of precision for estimating the CR rate at EOT. If 45 of 50 patients have a CR at EOT, the CR rate at EOT would be 90% with 95% confidence interval (CI) of 78.2% to 96.7%, based on the exact Clopper-Pearson method.30 The 73% CR rate at EOT observed in the A+AVD arm in the ECHELON-1 trial was used as the relevant benchmark when determining an appropriate sample size for this study.8
ORR, defined as the proportion of patients with a CR or a partial response at EOT, and the exact 2-sided 95% CIs using the Clopper-Pearson method were calculated. DOR was defined as the time from the first documentation of objective tumor response to the first documentation of progressive disease (PD), or death, whichever came first.29 DOCR was defined as time from start of the first documentation of CR to the first documentation of PD, or death, whichever came first. PFS was defined as time from start of study treatment to first documentation of objective PD or death. Patients without progression or death were censored on the date of the last radiological assessment. DOR, DOCR, and PFS were analyzed using the Kaplan-Meier method. DOR, DOCR, PFS rates, and the associated 95% CIs were calculated using complementary log-log transformation.31
Results
Patient disposition and baseline characteristics
Overall, 58 patients were enrolled; all but 1 patient received ≥1 dose of study drug. One patient withdrew consent before study drug administration. Patients were predominantly male (53%) and White (88%), with a median age of 35.0 years (Table 1). All enrolled patients were aged >18 years. Patients had an Eastern Cooperative Oncology Group performance status performance status of 0 (60%) or 1 (40%). No patients had stage I disease; patients had either stage II bulky (32%) or stage III/IV disease (68%).
Demographics
| Demographics . | Total (N = 57) . |
|---|---|
| Age (y), median (range) | 35.0 (19-78) |
| Age (y), range, n (%) | |
| <60 | 51 (89) |
| ≥60 | 6 (11) |
| Sex, n (%) | |
| Female | 27 (47) |
| Male | 30 (53) |
| Ethnicity, n (%) | |
| Hispanic | 9 (16) |
| Not Hispanic | 46 (81) |
| Unknown | 2 (4) |
| Race, n (%) | |
| White | 50 (88) |
| Black or African American | 2 (4) |
| Asian | 1 (2) |
| Multiple | 1 (2) |
| Unknown | 3 (5) |
| Disease stage at diagnosis, n (%) | |
| II | 18 (32) |
| Bulky∗ | 17 (30) |
| III | 10 (18) |
| IV | 29 (51) |
| Extranodal disease present at initial diagnosis, n (%) | 28 (49) |
| B symptoms present at initial diagnosis, n (%) | 33 (58) |
| International Prognostic Score, n (%) | |
| 0-1 | 13 (23) |
| 2-3 | 32 (56) |
| 4-7 | 12 (21) |
| Demographics . | Total (N = 57) . |
|---|---|
| Age (y), median (range) | 35.0 (19-78) |
| Age (y), range, n (%) | |
| <60 | 51 (89) |
| ≥60 | 6 (11) |
| Sex, n (%) | |
| Female | 27 (47) |
| Male | 30 (53) |
| Ethnicity, n (%) | |
| Hispanic | 9 (16) |
| Not Hispanic | 46 (81) |
| Unknown | 2 (4) |
| Race, n (%) | |
| White | 50 (88) |
| Black or African American | 2 (4) |
| Asian | 1 (2) |
| Multiple | 1 (2) |
| Unknown | 3 (5) |
| Disease stage at diagnosis, n (%) | |
| II | 18 (32) |
| Bulky∗ | 17 (30) |
| III | 10 (18) |
| IV | 29 (51) |
| Extranodal disease present at initial diagnosis, n (%) | 28 (49) |
| B symptoms present at initial diagnosis, n (%) | 33 (58) |
| International Prognostic Score, n (%) | |
| 0-1 | 13 (23) |
| 2-3 | 32 (56) |
| 4-7 | 12 (21) |
Defined as a mediastinal single node or nodal mass of ≥10 cm.
Efficacy
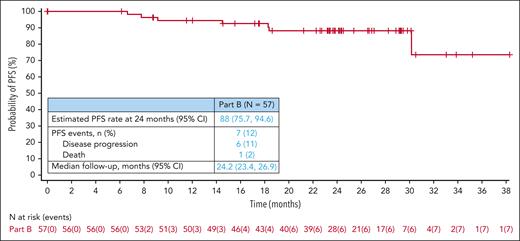
At the time of data cutoff (22 May 2023), the CR rate at EOT was 88% (n = 50/57; 95% CI, 76.3-94.9). The ORR at EOT was 93% (n = 53/57; 95% CI, 83.0-98.1; Table 2). A total of 88% (95% CI, 75.7-94.6) of responders had a DOR lasting >2 years. Of patients who achieved a CR, 88% (95% CI, 76.0-94.6) had a DOCR lasting >2 years; 6 patients relapsed. At a median follow-up of 24.2 months (95% CI, 23.4-26.9), the estimated 2-year PFS rate was 88% (95% CI, 75.7-94.6; Figure 1). Of 57 patients, 7 (12%) had a PFS event including 6 patients with PD and 1 patient who died because of sepsis, outside the safety reporting period. No patients received subsequent palliative or consolidative radiation therapy; all subsequent therapies were systemic regimens. One patient had an indeterminate response at EOT that converted to a CR during long-term follow-up. The estimated 2-year PFS rate for the subset of patients with stage II bulky (n = 17) and stage III/IV (n = 39) disease was 94% (95% CI, 63.2-99.1) and 85% (95% CI, 68.3-93.7), respectively (supplemental Figure 1).
Overall response at EOT
| Response . | All treated, (N = 57) . | Efficacy evaluable, (n = 56) . |
|---|---|---|
| Objective response rate (CR + PR), n (%) | 53 (93) | 53 (95) |
| 95% CI | (83.0-98.1) | (85.1-98.9) |
| CR, n (%) | 50 (88) | 50 (89) |
| 95% CI | (76.3-94.9) | (78.1-96.0) |
| PR, n (%) | 3 (5) | 3 (5) |
| 95% CI | (1.1-14.6) | (1.1-14.9) |
| Stable disease, n (%) | 0 | 0 |
| PD, n (%) | 2 (4) | 2 (4) |
| Indeterminate response, n (%) | 1 (2) | 1 (2) |
| Not evaluable, n (%) | 1 (2) | 0 |
| Proportion of patients with a DOR∗ (mo) beyond 2 y (95% CI), % | — | 88.3 (75.7-94.6) |
| Proportion of patients with a DOCR∗ (mo) beyond 2 y (95% CI), % | — | 88.4 (76.0-94.6) |
| Response . | All treated, (N = 57) . | Efficacy evaluable, (n = 56) . |
|---|---|---|
| Objective response rate (CR + PR), n (%) | 53 (93) | 53 (95) |
| 95% CI | (83.0-98.1) | (85.1-98.9) |
| CR, n (%) | 50 (88) | 50 (89) |
| 95% CI | (76.3-94.9) | (78.1-96.0) |
| PR, n (%) | 3 (5) | 3 (5) |
| 95% CI | (1.1-14.6) | (1.1-14.9) |
| Stable disease, n (%) | 0 | 0 |
| PD, n (%) | 2 (4) | 2 (4) |
| Indeterminate response, n (%) | 1 (2) | 1 (2) |
| Not evaluable, n (%) | 1 (2) | 0 |
| Proportion of patients with a DOR∗ (mo) beyond 2 y (95% CI), % | — | 88.3 (75.7-94.6) |
| Proportion of patients with a DOCR∗ (mo) beyond 2 y (95% CI), % | — | 88.4 (76.0-94.6) |
PR, partial response.
DOR/DOCR analyses are performed in patients who achieved a PR or CR (DOR)/CR (DOCR). Data cutoff: 22 May 2023.
Kaplan-Meier estimate of PFS, with estimated PFS rate at 2 years, PFS events, and median follow-up. One patient died from sepsis secondary to aspiration pneumonia and bacteremia ∼3.5 months after last dose of study drug.
Kaplan-Meier estimate of PFS, with estimated PFS rate at 2 years, PFS events, and median follow-up. One patient died from sepsis secondary to aspiration pneumonia and bacteremia ∼3.5 months after last dose of study drug.
Safety
All 57 (100%) patients treated with AN+AD had ≥1 treatment-emergent AE (TEAE), with treatment-related AEs (TRAEs) occurring in 56 (98%) patients (supplemental Table 1). The most common any grade TRAEs were nausea (n = 37/57 [65%]), fatigue (n = 28/57 [49%]), and peripheral sensory neuropathy (n = 25/57 [44%]); 19 (33%) patients experienced grade ≥3 TRAEs, most commonly alanine aminotransferase increased (11%) and neutropenia (9%; Table 3). Of patients who experienced treatment-related peripheral sensory neuropathy (n = 25/57 [44%]), most (n = 23/25) experienced grade 1 (21%) or grade 2 (19%) events. The remaining 2 patients had grade 3 PN. Among all 44 recorded PN events (patients may have experienced >1 event), 18 (41%) events were resolved, 2 (5%) events improved, and 24 (55%) events neither improved nor resolved at the time of data cutoff. Treatment-emergent PN, comprising of Medical Dictionary for Regulatory Activities Standardized MedDRA Queries PN terms, occurred in 56% of patients (supplemental Table 2). No febrile neutropenia occurred; 28 (49%) patients received G-CSF. No patients died because of TRAEs.
TRAEs of any grade (in more than 10% of patients) or grade 3 or higher (in more than 2% of patients)
| Preferred term . | Total, (N = 57) . | |
|---|---|---|
| Any grade . | Grade ≥3 . | |
| Any TRAEs, n (%) | 56 (98) | 19 (33) |
| Nausea | 37 (65) | 0 |
| Fatigue | 28 (49) | 2 (4) |
| Peripheral sensory neuropathy | 25 (44) | 2 (4) |
| Alopecia | 20 (35) | 0 |
| Diarrhea | 18 (32) | 1 (2) |
| Constipation | 15 (26) | 0 |
| Alanine aminotransferase increased | 9 (16) | 6 (11) |
| Headache | 9 (16) | 0 |
| Vomiting | 9 (16) | 0 |
| Stomatitis | 8 (14) | 0 |
| Aspartate aminotransferase increased | 7 (12) | 2 (4) |
| Decreased appetite | 7 (12) | 0 |
| Rash maculopapular | 7 (12) | 1 (2) |
| Dyspepsia | 6 (11) | 0 |
| Myalgia | 6 (11) | 0 |
| Neutropenia | 6 (11) | 5 (9) |
| Pyrexia | 5 (9) | 2 (4) |
| Pneumonitis | 3 (5) | 2 (4) |
| Colitis | 2 (4) | 2 (4) |
| Preferred term . | Total, (N = 57) . | |
|---|---|---|
| Any grade . | Grade ≥3 . | |
| Any TRAEs, n (%) | 56 (98) | 19 (33) |
| Nausea | 37 (65) | 0 |
| Fatigue | 28 (49) | 2 (4) |
| Peripheral sensory neuropathy | 25 (44) | 2 (4) |
| Alopecia | 20 (35) | 0 |
| Diarrhea | 18 (32) | 1 (2) |
| Constipation | 15 (26) | 0 |
| Alanine aminotransferase increased | 9 (16) | 6 (11) |
| Headache | 9 (16) | 0 |
| Vomiting | 9 (16) | 0 |
| Stomatitis | 8 (14) | 0 |
| Aspartate aminotransferase increased | 7 (12) | 2 (4) |
| Decreased appetite | 7 (12) | 0 |
| Rash maculopapular | 7 (12) | 1 (2) |
| Dyspepsia | 6 (11) | 0 |
| Myalgia | 6 (11) | 0 |
| Neutropenia | 6 (11) | 5 (9) |
| Pyrexia | 5 (9) | 2 (4) |
| Pneumonitis | 3 (5) | 2 (4) |
| Colitis | 2 (4) | 2 (4) |
In 8 (14%) patients that experienced a serious TRAE, the most common were pneumonitis (5%) and pyrexia (5%). Four patients experienced a TEAE leading to dose discontinuation of all study drugs, including pyrexia (n = 2), drug reaction with eosinophilia and systemic symptoms (n = 1), and hypophysitis (n = 1). TEAEs leading to discontinuation of brentuximab vedotin were observed in 7 (12%) patients, mostly grade 3 (n = 6/57 [11%]) events; 1 patient had a grade 2 TEAE. These events included peripheral sensory neuropathy (n = 2) and colitis, drug reaction with eosinophilia and systemic symptoms, hyperglycemia, hypophysitis, and pyrexia (n = 1 each). TEAEs led to discontinuation of nivolumab in 9 (16%) patients and delay in 10 (18%) patients.
Treatment-emergent immune-mediated AEs (IMAEs) occurred in 19 (33%) patients; most commonly hypothyroidism (n = 5/57 [9%]), and pneumonitis and rash maculopapular (n = 3/57 each [5%]; Table 4). Grade ≥2 IMAEs occurred in 15 (26%) patients, and grade ≥3 IMAEs occurred in 8 (14%) patients (supplemental Table 3). Overall, 38 (67%) patients received prophylactic steroid, mostly to prevent infusion-related reactions (n = 26/57 [46%]; supplemental Table 4). Median duration of immune-modulating medication for IMAEs was 12.5 days (range, 3-81). All IMAEs were managed according to the current guidelines32 and the nivolumab investigator’s brochure; a summary of medications used to treat IMAEs can be found in the data supplement (supplemental Table 5).
Treatment-emergent IMAEs of any grade (in more than 2% of patients) or grade 3 or higher (all events)
| Preferred term . | Total (N = 57) . | |
|---|---|---|
| Any grade . | Grade ≥3 . | |
| Patients with any event, n (%)∗ | 19 (33) | 8 (14) |
| Hypothyroidism | 5 (9) | 0 |
| Pneumonitis | 3 (5) | 1 (2) |
| Rash maculopapular | 3 (5) | 1 (2) |
| Alanine aminotransferase increased | 2 (4) | 1 (2) |
| Aspartate aminotransferase increased | 2 (4) | 1 (2) |
| Dermatitis acneiform | 2 (4) | 0 |
| Rash | 2 (4) | 0 |
| Autoimmune hepatitis | 1 (2) | 1 (2) |
| Colitis | 1 (2) | 1 (2) |
| Dermatitis atopic | 1 (2) | 0 |
| Hypophysitis | 1 (2) | 1 (2) |
| Rash morbilliform | 1 (2) | 1 (2) |
| Transaminases increased | 1 (2) | 1 (2) |
| Preferred term . | Total (N = 57) . | |
|---|---|---|
| Any grade . | Grade ≥3 . | |
| Patients with any event, n (%)∗ | 19 (33) | 8 (14) |
| Hypothyroidism | 5 (9) | 0 |
| Pneumonitis | 3 (5) | 1 (2) |
| Rash maculopapular | 3 (5) | 1 (2) |
| Alanine aminotransferase increased | 2 (4) | 1 (2) |
| Aspartate aminotransferase increased | 2 (4) | 1 (2) |
| Dermatitis acneiform | 2 (4) | 0 |
| Rash | 2 (4) | 0 |
| Autoimmune hepatitis | 1 (2) | 1 (2) |
| Colitis | 1 (2) | 1 (2) |
| Dermatitis atopic | 1 (2) | 0 |
| Hypophysitis | 1 (2) | 1 (2) |
| Rash morbilliform | 1 (2) | 1 (2) |
| Transaminases increased | 1 (2) | 1 (2) |
One patient may have multiple events.
Discussion
In this phase 2 study of AN+AD in treatment-naive patients with stage II bulky or stage III/IV cHL, the CR rate and ORR at EOT were 88% and 93%, respectively. Responses to the AN+AD regimen were durable, with 88% of responders having DOR and DOCR of >2 years. The 2-year PFS rate was 88%, and no patients received subsequent radiation therapy.
Determining the optimal therapeutic approach for patients with stage I/II bulky disease is challenging, because many studies of cHL, including this study, report combined results for stage I/II bulky disease and III/IV disease. This preliminary data may support alternatives to currently available regimens for prognostically unfavorable early-stage disease leading to treatment algorithms that avoid the use of the chemotherapeutic regimen eBEACOPP followed by consolidation involved-field radiation therapy (IFRT),33 which is the treatment sequence used in other protocols. Results from this study support novel combinations for treating cHL, such as PD-1 inhibitor and CD30 antibody–drug conjugate combination regimens, given the comparable efficacy to other regimens.
Although limited by small sample size (n = 17), the 2-year PFS rate was 94% with AN+AD in patients with treatment-naive stage II bulky disease. In the well-established HD8 study in patients with newly diagnosed, bulky stage I/II and IIIA unfavorable disease evaluating 2 cycles of COPP (cyclophosphamide, vincristine, procarbazine, and prednisone) alternating with 2 cycles of the chemotherapy regimen ABVD followed by 30 Gy of extended-field or IFRT with 10 Gy to initial bulky disease, the 5-year freedom-from-failure rates ranged from 84% to 86%.34 In the phase 3 H10 trial in treatment-naive stage I/II disease evaluating an early PET-adapted approach after 2 cycles of ABVD, the 5-year PFS rates were 90% and 92% for patients with unfavorable PET-negative disease treated with either 4 additional cycles of ABVD alone or 2 cycles of ABVD followed by involved-node radiotherapy, respectively; 19% of patients had PET-positive disease, with 5-year PFS rates of 77% and 91% for patients treated with either 2 cycles of ABVD or 2 cycles of eBEACOPP followed by involved-node radiotherapy, respectively.35 In the phase 2 CALGB 50801 study, a PET-adapted strategy in treatment-naive patients with bulky stage I/II disease treated with 2 cycles of ABVD was evaluated. Patients with interim PET2-negative disease continued to receive ABVD for 4 cycles, whereas those with PET2-positive (Deauville score of 4-5) disease were escalated to eBEACOPP for 4 cycles with 30.6 Gy of IFRT. Most patients completed 6 cycles of ABVD; 22% of patients escalated to receiving 4 cycles of eBEACOPP followed by 30.6 Gy IFRT, with a 3-year PFS rate of 90%.33 The preliminary data for the AN+AD immuno-oncology (IO) combination regimen may be useful to investigators interested in exploring alternatives to previously tested first-line combination chemotherapeutic regimens with or without radiotherapy. IO combination regimens, such as PD-1 inhibitors with antibody-drug conjugates, should be further explored as frontline options for this challenging subset of patients with unfavorable, early-stage cHL.
In patients with stage III/IV disease treated with AN+AD (n = 39), the 2-year PFS rate was 85%, similar to what was observed with A+AVD in ECHELON-1, despite differences in sample sizes (n = 39 vs 664).8 In the phase 3 ECHELON-1 study evaluating up to 6 cycles of the IO regimen A+AVD vs the chemotherapeutic regimen ABVD for patients with treatment-naive stage III/IV disease, the 2-year modified PFS rates were 82% vs 77%, with ORR rates of 86% vs 83%.8 Both the A+AVD and AN+AD regimens include brentuximab vedotin, doxorubicin, and dacarbazine; however, A+AVD includes vinblastine and not nivolumab.
In the phase 3 RATHL study evaluating a PET-adapted approach in patients with bulky stage IIA or IIB/IV disease after 2 prior cycles of ABVD, those with interim PET-negative disease (84%) who received ABVD or AVD (no bleomycin) for an additional 4 cycles had 3-year PFS rates of 86% and 84%, respectively; 14% of eligible patients with interim PET-positive disease (Deauville score of 4/5) were given either BEACOPP or eBEACOPP, with a 3-year PFS rate of 68%.26
Recently, the phase 3 GHSG HD21 trial assessing BrECADD vs escalated BEACOPP in patients with stage IIB with risk factors or stage III to IV disease demonstrated a superior 4-year PFS rate with the BrECADD regimen (94% vs 91%, respectively), showing an improvement from eBEACOPP.24 Both the BrECADD regimen and the AN+AD regimen used in the current study contain brentuximab vedotin, doxorubicin, and dacabarzine; however, AN+AD includes nivolumab, and BrECADD includes the chemotherapeutic agents etoposide, cyclophosphamide, and dexamethasone. In another recent presentation from the phase 3 SWOG S1826 study evaluating 6 cycles of N+AVD or A+AVD in patients with stage III-IV disease without regards for PET adaptation, 1-year PFS rates were 94% vs 86%.23 N+AVD has become a commonly adapted regimen in countries where ABVD has been a SOC in the past. The AN+AD, N+AVD, and A+AVD regimens all contain doxorubicin and dacarbazine; the only difference between AN+AD and N+AVD is the inclusion of brentuximab vedotin in AN+AD and vinblastine in N+AVD. Direct comparisons between these trials are not possible, considering key differences. ECHELON-1 and SWOG S1826 are limited to patients with stage III/IV disease; the eligibility of patients for this trial, the RATHL, and GHSG HD21 trials include patients with early-stage disease. The SWOG S1826 trial also included pediatric (aged ≥12 years) patients, whereas the other trials did not; the GHSG HD21 trial was limited to patients aged ≤60 years. The results with AN+AD presented here may be useful in determining future combinations for frontline therapy, as patients may prefer IO combinations with anti–PD-1 antibodies and CD30 antibody–drug conjugates.
In this study, AN+AD demonstrated a manageable safety profile. Only 44% (n = 25) of patients experienced treatment-related peripheral sensory neuropathy; 92% of those cases being grades 1 (21%) or 2 (19%), which is numerically lower than has been reported in other frontline studies of IO combinations. Considering the limitations of cross-trial comparisons, the incidence and severity of PN in patients treated with A+AVD in the ECHELON-1 and SWOG S1826 studies was higher than what was observed in this study, with incidences of 67% (grade ≥3, 11%) and 63% (grade ≥3, 9%), respectively.8,23 No cases of febrile neutropenia were reported with AN+AD in this study; 49% of patients received G-CSF, all for primary prophylaxis. In ECHELON-1, febrile neutropenia was reported in 19% (all grade ≥3) of patients treated with A+AVD; rates of febrile neutropenia were 11% and 21% with and without administration of prophylactic G-CSF, respectively. G-CSF prophylaxis was recommended for patients receiving A+AVD after 75% of patients were already enrolled. In the SWOG S1826 study, febrile neutropenia occurred in 7% of patients treated with A+AVD, 95% of whom received G-CSF prophylaxis.23 In this study with AN+AD, treatment-related neutropenia occurred in 11% (grade ≥3, 9%) of patients; neutropenia occurred in 58% (grade ≥3, 54%) and 32% (grade ≥3, 25%) of patients receiving A+AVD in ECHELON-1 and SWOG S1826, respectively.8,23
Direct comparison of AN+AD with the N+AVD arm of the SWOGS186 study is not possible, but AN+AD demonstrated favorable rates of neutropenia, in contrast to what was observed in the SWOGS186 study, with rates of 55% (grade ≥3, 47%) observed in patients treated with N+AVD.23 Treatment with G-CSF was optional in the SWOG S1826 study with N+AVD, with 54% of patients receiving G-CSF. Rates of febrile neutropenia were similar, occurring in 0% and 5% of patients treated with AN+AD or N+AVD, respectively. Higher rates of PN were observed with AN+AD vs N+AVD, with 44% (grade ≥3, 4%) and 33% (grade ≥3, 1%) of patients experiencing this event, respectively. Most common immune-mediated TEAEs in patients treated with AN+AD were hypothyroidism (any grade, 9%; grade ≥3, 0%), pneumonitis (any grade, 5%; grade ≥3, 2%), and rash maculopapular (any grade, 5%; grade ≥3, 2%). In patients treated with N+AVD, the most common immune or other AEs of interest were increased alanine aminotransferase (any grade, 32%; grade ≥3, 5%), increased aspartate aminotransferase (any grade, 25%; grade ≥3, 2%), rash maculopapular (any grade, 11%; grade ≥3, 1%), and hypothyroidism (any grade, 7%; grade ≥3, 0%). Therefore, the profiles of immune-related events differed between the 2 regimens, with higher proportions of patients receiving N+AVD experiencing increased alanine aminotransferase (32% vs 4%) and aspartate aminotransferase (25% vs 4%).
Both the A+AVD and N+AVD regimens include vinblastine, which is known to be associated with neuropathy and myelosuppression. The higher levels of toxicities associated with brentuximab vedotin and vinblastine in combination may be because of their overlapping MOAs targeting microtubule formation.10 Substituting antibody–drug conjugates such as brentuximab vedotin for vinblastine may provide an intriguing treatment option for cHL in combination with PD-1 inhibitors.
In addition, both the A+AVD and N+AVD regimens also included dacarbazine, which has a boxed warning for hepatic necrosis. Because vinblastine is primarily metabolized by the liver, the combination of these 2 drugs may result in increased hepatotoxicity. The rates of immune-related increased alanine aminotransferase and aspartate aminotransferase were higher in patients treated with A+AVD and N+AVD than in those treated with AN+AD. Thus, exclusion of vinblastine in combination regimens with dacarbazine may result in improvements in liver toxicities.
Overall, patients treated with AN+AD had a potentially favorable safety profile.8,15,23 Limitations of this study include a small sample size (n = 57) with enrollment exclusively in the United States. Additionally, because this study was noncomparative in nature, direct comparisons to the current SOC or other studies with a larger patient sample size cannot be made.
Historically, combination chemotherapy with or without radiation has been the SOC for patients with cHL. Incorporation of IO agents, such as brentuximab vedotin and nivolumab, into treatment has revolutionized management of cHL, as studies support frontline therapy with these agents.36 This shift from intense combination chemotherapy regimens to IO-based regimens has resulted in improved efficacy and, potentially, safety in patients with cHL, although there is still a need to further improve safety and quality of life. Previous clinical trials have demonstrated durable efficacy and limited toxicity in patients treated with brentuximab vedotin and nivolumab in relapsed/refractory cHL (CR rate, 67%; ORR, 85%) and as a frontline treatment in elderly patients (median age, 72 years) that are unable to tolerate traditional chemotherapy regimens (CR rate, 67%; ORR, 86%; median follow-up, 51.6 months).17,18 Therefore, combining IO agents, such as antibody-drug conjugates and PD-1 inhibitors, with chemotherapy may eliminate the need for vinblastine and bleomycin in treating cHL. AN+AD continues to show a tolerable safety profile, with no new safety signals, along with a high CR rate, ORR, and encouraging DOR that will continue to evolve with long-term follow-up. These results warrant further investigation of PD-1 inhibitor and CD30 antibody–drug conjugate combination regimens in a larger study in advanced cHL.
Acknowledgments
The authors thank the patients who participated in this study, their families, and the investigators and staff at SGN35-027 clinical study sites, and the members of the safety monitoring committee. The authors also thank Griffith Davis and Marianna Johnson at Pfizer for their critical input into and review of an earlier version of the manuscript. The authors acknowledge the medical monitoring of the study and the entire SGN35-027 study team.
Medical writing support and editorial assistance were provided by Hanna Thomsen (funded by Seagen Inc, acquired by Pfizer in December 2023), and Brittany Woodby of The Nucleus Group Holdings, Inc, and was funded by Pfizer. This study was sponsored by Seagen Inc, Bothell, Washington (acquired by Pfizer in December 2023); Takeda Development Center Americas, Inc, Lexington, Massachusetts; and Bristol Myers Squibb, Inc, Princeton, New Jersey.
The views expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, the Department of Defense, or the US government.
Authorship
Contribution: All authors contributed to the conception and design of the study, provision of study materials or patients, collection and assembly of data, data analysis and interpretation, manuscript writing and final approval of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: H.J.L. serves as a consultant to Century Therapeutics; received honoraria from Aptitude Health, Cancer Experts, Curio Sciences, Deloitte, Guidepoint, Janssen, Korean Society of Cardiology, and Olson Research; and reports research funding/grants from Bristol Myers Squibb (BMS), Celgene, Oncternal Therapeutics, Pharmacyclics, Seagen (acquired by Pfizer in December 2023), and Takeda. R.R. serves as a consultant to BMS, Merck, Pharmacyclics, and Seagen (acquired by Pfizer in December 2023) and reports research funding/grants from Merck, Pharmacyclics, Seagen (acquired by Pfizer in December 2023), Curis, Trillium, and Cellectar. J.F. reports stock ownership in Amgen, Abbott, and AbbVie and serves on a speakers bureau for Jazz Pharmaceuticals and AstraZeneca. J.M. serves on a speakers bureau for AstraZeneca and Janssen. I.W.F. serves as a consultant for AbbVie, BeiGene, Genentech, Genmab, Kite Pharma, Roche, and Vincerx Pharma; reports research grants from AbbVie, Acerta Pharma, Agios, ArQule, AstraZeneca, BeiGene, Biopath, BMS, Calibr, CALGB, Celgene, City of Hope National Medical Center, Constellation Pharmaceuticals, Curis, CTI Biopharma, Epizyme, Fate Therapeutics, Forma Therapeutics, Forty Seven, Genentech, Gilead Sciences, InnoCare Pharma, IGM Biosciences, Incyte, Infinity Pharmaceuticals, Janssen, Kite Pharma, Loxo, Marker Therapeutics, Merck, Millennium Pharmaceuticals, MorphoSys, Myeloid Therapeutics, Novartis, Nurix, Pfizer, Pharmacyclics, Portola Pharmaceuticals, Rhizen Pharmaceuticals, Roche, Seattle Genetics, Step Pharma, Tessa Therapeutics, TG Therapeutics, Trillium Therapeutics, Triphase Research and Development Corporation, Unum Therapeutics, Verastem, Vincerx Pharma, and 2seventy bio; and serves on an advisory committee for Vincerx. J.M.B. serves on a speakers bureau for BioGene; is a consultant to AbbVie, Adaptive Biotechnologies, ADC Therapeutics (ADCT), AstraZeneca, BeiGene, BMS, Constellation, Eli Lilly, Foresight, Genentech, Genmab, Kura, Kymera, MorphoSys, Novartis, Nurix, Regeneron, Seagen (acquired by Pfizer in December 2023), and TG Therapeutics; and reports research funding/grants from MorphoSys. Y.L. serves as an advisory board member for Pfizer, AbbVie, Spectrum, Genmab, TG Therapeutics, Seagen (acquired by Pfizer in December 2023), BeiGene, Gilead, BMS, and Genentech; is a consultant to Pfizer; serves on the speakers bureau for Kyowa; and reports research funding/grants from BeiGene, ADCT, Genentech, and Seagen (acquired by Pfizer in December 2023). M.P. was previously employed at Brooke Army Medical Center. M.R. serves as an advisory board member for AstraZeneca, Alexion, Sanofi Genzyme, BMS, ADCT, GlaxoSmithKline (GSK), Adaptive, Epizyme, Agios, PharmaEssentia, Takeda, and BeiGene; is a consultant to Takeda, Sanofi Genzyme, and GSK; serves on a speakers bureau for Takeda, AstraZeneca, AbbVie, Alexion, Epizyme, Ipsen, Astellas, Incyte/MorphoSys, BeiGene, ADCT, Sanofi, Adaptive, CTI, GSK, Genmab, and BMS; reports research funding/grants from Amgen, Genentech, Seagen (acquired by Pfizer in December 2023), Merck, Janssen, AstraZeneca, AbbVie, Sanofi Genzyme, Novartis, GSK, Epizyme, Karyopharm, and MorphoSys. R.C. serves as an advisory board member for AbbVie, AstraZeneca, Daiichi Sankyo, BMS, Kite Pharmaceuticals, MorphoSys, Janssen, Pfizer, Biotheranostics, and GSK and received honoraria from Curio Sciences, Amerisource Bergen, Cardinal health, and MJH Life Sciences. T.A.F. serves as an advisory board member for BMS, Seagen (acquired by Pfizer in December 2023), Genmab, and AstraZeneca; reports travel expenses from AbbVie, Kite Pharma, Seagen (acquired by Pfizer in December 2023), and Takeda; serves as a consultant to AstraZeneca, BMS, MorphoSys, and Seagen (acquired by Pfizer in December 2023); serves on a speakers bureau for AbbVie and Seagen (acquired by Pfizer in December 2023); reports research funding and grants from Amgen, BMS, Celgene, Cell Medica, Corvus, Eisai, Kyowa Hakko Kirin, Pfizer, Portola Pharma, Roche, Seagen (acquired by Pfizer in December 2023), Trillium, and Viracta; and received honoraria from AbbVie, BMS, Kite Pharma, Pharmacyclics, Seagen (acquired by Pfizer in December 2023), Takeda, and Genmab. H.Y. serves on a speakers bureau for AbbVie, AstraZeneca, BeiGene, GlaxoSmithKline, Janssen Biotech, Karyopharm Therapeutics, and Takeda. M.I.-O. serves as an advisory board member for ADCT, Kite, Jansen Biotech, and Incyte. A.P. serves on an advisory board for Janssen and AbbVie. L.M. serves on a speakers bureau for Incyte and Taiho Oncology; and serves on the scientific advisory board for Pfizer. A.D. serves on a speakers bureau for Janssen/Pharmacyclics, AVEO, Lilly, Seagen (acquired by Pfizer in December 2023), and Astellas. V.R. received honoraria from MJH Holdings and Curio Sciences. M.D.G. serves as an advisory board member for Janssen Oncology and Sanofi. J.R. serves as an advisory board member for Genentech; serves on a speakers bureau for Amgen, AbbVie, Karyopharm, AstraZeneca, and BeiGene; reports employment at Texas Oncology; and serves as a consultant to Seagen (acquired by Pfizer in December 2023), Novocure, AADI, and AbbVie. L.H., M.A.F., and W.G. report equity ownership and employment for Pfizer, Inc. C.A.Y. serves on a speakers bureau for BeiGene and as a consultant to Seagen (acquired by Pfizer in December 2023) and reports research funding/grants from Seagen (acquired by Pfizer in December 2023). P.G. declares no competing financial interests.
The current affiliation for M.I.-O. is St. Elizabeth Physicians Cancer Center, Edgewood, KY.
The current affiliation for R.R. is University of Pittsburgh Medical Center, Pittsburgh, PA.
Correspondence: Hun Ju Lee, The Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Unit 429, 1515 Holcombe Blvd, Houston, TX 77030; email: hunlee@mdanderson.org.
References
Author notes
Presented at the 2023 American Society of Hematology Annual Meeting and Exposition, San Diego, CA, 10 December 2023.
Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal