In this issue of Blood, Nair et al, in the article entitled “Heme promotes venetoclax resistance in multiple myeloma through MEK-ERK signaling and purine biosynthesis,” show that heme, a natural metabolite, can influence the response to the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax in multiple myeloma (MM) cells.1
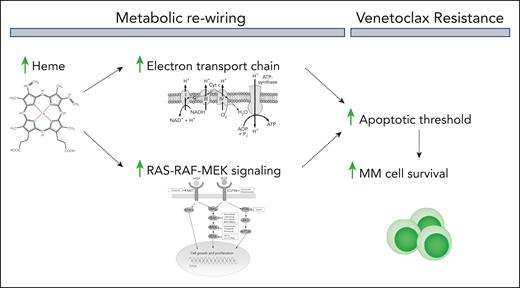
Heme regulates the electron transport chain (ETC), which resides in the mitochondria and produces adenosine triphosphate to support the energy needs of cells. Reduced ETC activity leads to mitochondrial dysfunction and decreased oxidative phosphorylation (OXPHOS), which lowers the apoptotic threshold, making MM cells more vulnerable to venetoclax (see figure).2
Heme enhances electron transport chain activity and promotes the activation of the growth-promoting RAS-RAF-MEK signaling cascade. This results in a metabolic reprogramming that elevates the apoptotic threshold of the cells, thereby inhibiting venetoclax-induced MM cell death.
Heme enhances electron transport chain activity and promotes the activation of the growth-promoting RAS-RAF-MEK signaling cascade. This results in a metabolic reprogramming that elevates the apoptotic threshold of the cells, thereby inhibiting venetoclax-induced MM cell death.
Expanding on this prior research, Nair et al now report that venetoclax-sensitive MM cells have inherently reduced heme biosynthesis. As if to make up for this shortfall in heme, these cells have increased hemin (oxidized heme) uptake—likely scavenging it from the diet or lysed red blood cells. Furthermore, they show that hemin supplementation reverses the cell sensitivity to venetoclax and promotes a shift toward increased de novo purine synthesis. Conversely, targeting ferrochetalase, a key enzyme in the heme biosynthesis pathway, further increases the sensitivity to venetoclax of both cell lines and primary cells from patients with MM. These results echo those found in acute myeloid leukemia (AML), where reducing heme biosynthesis potentiates apoptosis through the loss of ETC activity, resulting in baseline depolarization of the mitochondrial membrane and an increased propensity to undergo apoptosis.3
Metabolic and apoptotic signaling are intrinsically interconnected, and these interactions could be therapeutically exploited to design combination therapies or identify cancers with metabolic alterations, predisposing them to apoptosis.4 Indeed, several studies have demonstrated that the effectiveness of BH3 mimetics—drugs like venetoclax that neutralize the function of anti-apoptotic proteins—is significantly enhanced in MM and other blood cancers when combined with therapies that target mitochondrial metabolism. For instance, inhibiting glutamine metabolism augments the apoptosis-primed state of cells, thereby increasing their sensitivity to BH3 mimetics.5 Chen et al, in 2019, further demonstrated that disrupting mitochondrial function and dynamics sensitizes AML to venetoclax.6 Furthermore, combining venetoclax with azacitidine has significant therapeutic efficacy in patients with de novo AML, effectively eradicating leukemic stem cells by impairing OXPHOS.7 These studies illustrate how understanding the metabolism of relapsed/refractory cancer cells may help the design of combination therapies to overcome resistance.
Mechanistically, Nair et al report that activation of the MEK-ERK signaling cascade contributes to heme-mediated resistance to venetoclax (see figure). The ERK signaling pathway is a key regulator of both expression and functional activity of BCL2 family proteins, and its activation has been observed in venetoclax-resistant cancer cells.8,9 Moreover, hemin-supplemented cells display increased expression of myeloid cell leukemia 1 (MCL1) and heightened mitochondrial sensitivity to the MCL1 peptide MS-1.
Although Nair et al found that hemin-mediated reversal of venetoclax sensitivity was generally specific to venetoclax and did not mitigate the cytotoxic effects of other standard MM therapies, they observed that in 1 cell line, hemin supplementation induced resistance to both bortezomib and melphalan. This raises the possibility that this phenomenon may apply to other apoptosis-inducing therapies. It is possible that primary MM cells that do not downregulate heme biosynthesis are inadequately primed for apoptosis, potentially increasing the risk of therapy failure in these patients. Indeed, by analyzing paired samples of patients with newly diagnosed and relapsed/refractory MM, the authors found gene signatures associated with heme biosynthesis, as well as purine and pyrimidine metabolism to be elevated in the relapsed/refractory samples. This suggests a broader role for increased heme metabolism in the progression of MM and its impact on therapy sensitivity and resistance, underscoring the metabolic reprogramming occurring in resistant patients. Future research should aim to clarify if the activity of the heme pathway could function as a molecular biomarker to identify patients who are more likely to respond to cytotoxic, apoptosis-inducing therapy. In this regard, exploring how personalized metabolic data can guide cancer treatment will be essential.
In conclusion, Nair et al have uncovered the role of heme as a metabolic vulnerability whose disruption can potentiate myeloma cell death induced by selective BCL-2 inhibition. As we refine the clinical use of venetoclax in MM,10 a pivotal question is whether targeting heme could offer a more flexible and less toxic strategy to overcome resistance to venetoclax, ultimately improving patient outcomes.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal