In this issue of Blood, Vogl et al report the results of a first-in-human study of modakafusp alfa in patients with advanced relapse and refractory multiple myeloma (RRMM) with ≥3 prior lines of treatment, including at least 1 proteasome inhibitor (PI) and 1 immunomodulator drug (IMiD).1 By delivering interferon alfa signaling to CD38+ myeloma tumor cells, modakafusp alfa induced myeloma cell death while enhancing immune activation of immune cells carrying CD38+ and interferon alfa receptors.
Interferons are cytokines released predominantly by immune and stromal cells to affect a multitude of cellular responses, mainly the activation of cellular components of the immune response, such as dendritic cells, macrophages, and T cells,2 and promote antitumor immune responses. Interferon-based anticancer immunotherapy was unsuccessfully studied in the past in multiple myeloma, and despite clear activity, it was stopped because of significant toxicity.3
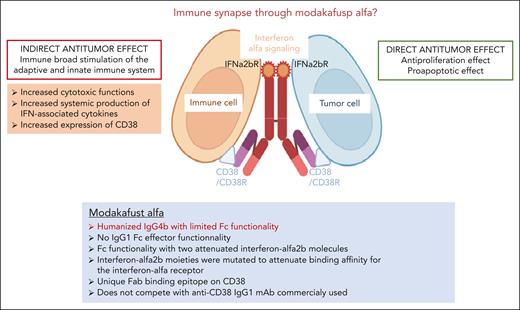
The modakafusp alfa is a new immunocytokine-based immunotherapy composed of a humanized IgG4 antibody structured with an anti-CD38 Fab portion with limited Fc functionality and 2 attenuated interferon alfa 2b molecules. Modakafusp alfa was designed to induce interferon signaling in CD38-expressing cells, including both myeloma tumor cells and immune cells. The interferon alfa 2b moieties were mutated to attenuate binding affinity for the interferon alfa receptor, thus allowing highly specific delivery of interferon to CD38+ cells.4 Interestingly, modakafusp alfa binds to CD38 at a different epitope than current commercialized anti-CD38 therapeutic antibodies.
Modakafusp alfa has a novel immune dual mode of action, through its interferon alfa signaling, with direct antiproliferative and proapoptotic effects on myeloma cells,5 and promotes in parallel an indirect mechanism of action on the immune niche directed toward control of the tumor mass, resulting in natural killer (NK) and CD8 T-cell activation, proliferation, and enhanced cytotoxic function, as well as systemic production of interferon-associated cytokines. Upregulation of CD38 expression on NK, T, and myeloma cells has also been described, consistent with the CD38 gene-containing interferon response elements in its promoter region (see figure).
Modakafusp alfa immunocytokine fusion protein. Novel immune mechanisms of action. Modakafusp alfa immunocytokine-induced myeloma cell death by delivering interferon alfa signaling to CD38+ myeloma tumor cells and enhancing immune activation by signaling to immune cells carrying CD38+ and interferon alfa receptors. IFN, interferon; IFNa2bR, interferon alfa-2b receptor; mAb, monoclonal antibody.
Modakafusp alfa immunocytokine fusion protein. Novel immune mechanisms of action. Modakafusp alfa immunocytokine-induced myeloma cell death by delivering interferon alfa signaling to CD38+ myeloma tumor cells and enhancing immune activation by signaling to immune cells carrying CD38+ and interferon alfa receptors. IFN, interferon; IFNa2bR, interferon alfa-2b receptor; mAb, monoclonal antibody.
Here, the authors report on dose escalation of patients with RRMM with a median of 6.5 lines of prior therapy; 83% had disease refractory to an IMiD, a PI, and an anti-CD38 antibody (triple class refractory). The feasible dosing schedule was every 4 weeks (Q4W) with a maximum tolerated dose of 3 mg/kg. Among the 30 patients treated at 1.5 mg/kg Q4W, the overall response rate (ORR) was 43.3%, with a median duration of response of 15.1 months (95% confidence interval [CI], 7.1-26.1 months); the median progression-free survival was 5.7 months (95% CI, 1.2-14.0 months). For patients without prior anti–B-cell maturation antigen (BCMA) therapy, the ORR was 58%. A lower ORR of 28% was observed among patients with prior BCMA-targeted therapy; however, these patients had received more extensive overall prior therapy (median, 9 vs 5 prior lines among our BCMA-naïve patients). Grade ≥3 adverse events occurred in 93.3% of patients, with primarily hematologic events, neutropenia (66.7%), and thrombocytopenia (46.7%) being the most common, with a low rate of infections reported in 26.7% patients. Importantly, the authors did not observe any neuropsychiatric and constitutional adverse effects typically seen with systemic interferon alfa therapy.
In summary, the modakafusp alfa is a new type of immunotherapy drug that demonstrated novel mechanisms of action, inducing upregulation of type I interferon signaling, increasing CD38 receptor density on target cells, and activating innate and adaptive immunity. The immune-stimulatory effects of modakafusp alfa suggest that there may be the potential of combination with antimyeloma therapies, including anti-CD38 antibodies, and more recent immunotherapies, such as T-cell–redirecting agents. Given the recent demonstration that immunotherapy combinations with proteasome inhibitors, immunomodulator drugs, or other immune approaches are feasible with manageable toxicities and enhanced efficacies, modakafusp alfa is likely to play a key role in the future in the treatment of multiple myeloma.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal