Everyone knows stories about uninvited guests who crashed the party or pesky flatmates who messed up the kitchen and never cleaned the bathroom. Therefore, it feels good to have roommates who make sure that no one goes overboard. In this issue of Blood, Isojima et al1 show that similar things happen in the bone marrow, where immature granulocyte (neutrophil) precursors not only are mere residents of the bone apartment but, in fact, ensure that the mansion is not destroyed by the uncontrolled growth of annoying roommates, the bone-resorbing osteoclasts.
Hematologists and immunologists would agree on the notion that circulating neutrophils, generated from immature precursors in the bone marrow and recruited to sites of inflammation to fight infections, are absolutely essential for survival.2 However, hyperactivated inflammatory neutrophils can also have detrimental side effects, such as the destruction of secondary lymphatic organs by the production of neutrophil extracellular traps (NETs).3 Indeed, NETs and the production of proinflammatory cytokines by neutrophils can also lead to peripheral bone destruction, as seen in periodontitis4 or rheumatoid arthritis.5 But although bone destruction by mature neutrophils is an accepted fact, there are also reports that the strong reduction or complete absence of neutrophils, for example, in severe congenital neutropenia, leads to bone loss and osteoporosis in humans6 or mice.7 Hence, under some circumstances, neutrophils seem to have bone protective effects but neither the involved neutrophil subtypes, nor the mechanisms were clear until now.
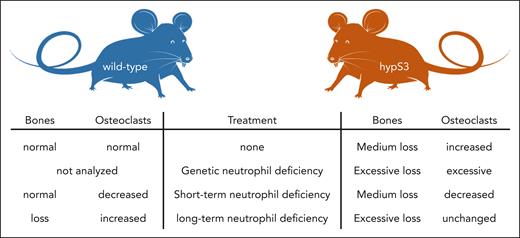
Isojima et al worked on a mouse model with osteoclast- and osteocyte-specific hyperactive STAT3 (hypS3), a transcription factor that mediates the effects of proinflammatory cytokines like interleukin 6. These mice already have a phenotype of above normal bone resorption. But when hypS3 mice were crossed with mice with a loss of colony stimulating factor 3 receptor (Csf3r), the receptor for the neutrophil-inducing cytokine granulocyte colony-stimulating factor, the bone loss phenotype was excessive because these double-mutated mice had very low neutrophil counts. A molecular analysis of the bone marrow of these mice using proteomics showed a profound loss of immature neutrophils in the double-mutated mice with hematopoietic development stopping at the preneutrophil stage. This finding was further corroborated by mapping the proteome data onto single-cell RNA sequencing data of the mouse bone marrow. To recapitulate the genetic loss of neutrophils in the double-mutated mice, they depleted them in the hypS3 model by 2 weeks of injection of the antibody 1A8, which binds to the neutrophil-specific Ly6G protein and rapidly destroys all mature neutrophils in mice.8 However, although this treatment led to a reduction in osteoclast transcripts in these mice, there was no obvious effect on the bone mass. The authors could explain this unexpected observation by the new finding that 1A8 treatment led to a marked increase in immature neutrophils in the bone marrow, while leaving preneutrophils untouched. Injection of 1A8 is only effective for ∼2 weeks, after which neutrophils are resistant to depletion,9 but a combination of 1A8 and a secondary anti-1A8 antibody can maintain depletion for weeks. Indeed, 6 weeks of treatment led to massive bone loss in wild-type and also hypS3 mice, and this was because of increased osteoclast-mediated bone resorption, not reduced bone formation. Bone destruction was mediated by both reduction of trabecular bone mass in the metaphyses and delayed cortical bone maturation in the diaphyses. The authors finally showed that long-term 1A8 treatment indeed reduced preneutrophils but did not increase immature neutrophils. This allowed the authors to directly demonstrate the profound negative impact of preneutrophils (which can be kept in culture, as opposed to immature neutrophils which cannot) on the development of osteoclasts from precursors in vitro. Hence, preneutrophils and immature neutrophils can inhibit the formation of osteoclasts, and this function can explain why early neutrophils maintain bone integrity and why durable profound loss of neutrophils is associated with bone loss (see figure).
Mice with hyperactive bone cells including osteoclasts (hypS3 mice) show strong bone loss and have increased osteoclast numbers. When hypS3 mice also suffer genetic neutrophil loss, their bone loss becomes excessive, and their osteoclast numbers are very high. This can be recapitulated in wild-type mice by antibody-mediated neutrophil depletion. But a short-term (2-week) depletion has the unexpected effect of saving bone. This is because of increases in immature bone marrow neutrophils by the treatment. Only long-term (6-week) antibody-mediated neutrophil loss reduces preneutrophils and leads to bone loss in wild-type and exaggerated bone loss in hypS3 mice. Hence, preneutrophils and immature neutrophils control bone loss by inhibiting osteoclastogenesis.
Mice with hyperactive bone cells including osteoclasts (hypS3 mice) show strong bone loss and have increased osteoclast numbers. When hypS3 mice also suffer genetic neutrophil loss, their bone loss becomes excessive, and their osteoclast numbers are very high. This can be recapitulated in wild-type mice by antibody-mediated neutrophil depletion. But a short-term (2-week) depletion has the unexpected effect of saving bone. This is because of increases in immature bone marrow neutrophils by the treatment. Only long-term (6-week) antibody-mediated neutrophil loss reduces preneutrophils and leads to bone loss in wild-type and exaggerated bone loss in hypS3 mice. Hence, preneutrophils and immature neutrophils control bone loss by inhibiting osteoclastogenesis.
The article describes and partially explains an unexpected and previously puzzling function of neutrophils in bone stability. This is important, because it focuses our attention on the significance of neutrophils for basic organismal physiology. What needs to be clarified next are the molecular factors that mediate this effect. The authors could show that osteoclast inhibition is mediated by diffusive components produced by preneutrophils but did not attempt to characterize them further. This, however, will be required if we are to make use of these interesting findings in the future, for example, when searching for alternative medications to treat bone loss in postmenopausal women. Some of the currently available medications can cause severe side effects.10 But it is very reassuring to know that we have these attentive flatmates that help to maintain our bone marrow homes.
Conflict-of-interest disclosure: M.G. reports no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal