In this issue of Blood, Wu et al1 study the function of circular RNA (circRNA) in platelet production. Following a trail of evidence that began with the observation that megakaryocytes and platelets express high levels of one particular circRNA, circFUT8, the team discovered that circFUT8 interacts with insulin-like growth factor 2 messenger RNA (mRNA) binding protein 2 (IGF2BP2) to promote the stabilization of tensin 1 (TNS1) mRNA (see figure). This mechanism exemplifies 1 function of circRNA, which is to modulate mRNA stability.
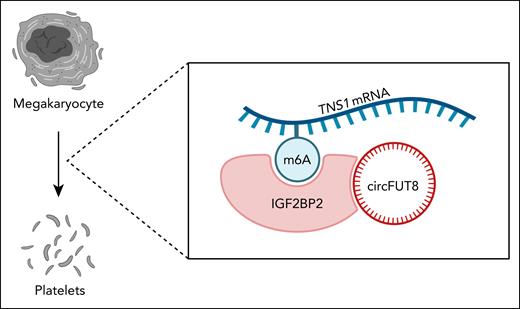
CircFUT8 interacts with IGF2BP2 to stabilize the TNS1 mRNA transcript in a N6-methyladenosine (m6A)–dependent manner. Wu et al demonstrate that this interaction contributes to TNS1 protein expression to regulate actin cytoskeletal rearrangement during platelet production. Figure created using BioRender.com. Moellmer S. (2025) https://BioRender.com/96bik04.
CircFUT8 interacts with IGF2BP2 to stabilize the TNS1 mRNA transcript in a N6-methyladenosine (m6A)–dependent manner. Wu et al demonstrate that this interaction contributes to TNS1 protein expression to regulate actin cytoskeletal rearrangement during platelet production. Figure created using BioRender.com. Moellmer S. (2025) https://BioRender.com/96bik04.
CircRNA forms through the process of back-splicing, in which a 5’ splice site joins with an upstream 3’ splice site to form a closed, single-stranded loop. The absence of exposed 3’ and 5’ ends makes circRNA resistant to degradation by exonucleases and thus more stable than linear RNA. CircRNA was discovered nearly 50 years ago in viruses,2 yet was considered functionally inconsequential to cellular organisms. The discovery of circRNA formed from pre-mRNA in eukaryotes,3 inspired researchers to circle back and explore the possibility that circRNAs play functional roles in cell biology. Once disregarded, circRNA has now been shown to regulate cell growth, development, and migration in a conserved manner from prokaryotes to eukaryotes. Although most of circRNAs are noncoding, a small subset of circRNA codes functional proteins, and noncoding circRNAs can modulate gene expression through interactions with mRNA or mRNA-binding proteins. The presence of thousands of unique circRNAs in humans has inspired those in translational research circles to use circRNAs as diagnostic biomarkers or therapeutic targets.
In hematopoietic stem cells, circRNA expression increases with cell maturation and varies between cell types.4 The differential expression of circRNAs can play a sinister role in hematopoiesis, with upregulation of circPAX5, circPVT1, and circHIPK3 associated with pediatric acute lymphoblastic leukemia5 and circBCL11B in acute myeloid leukemia pathogenesis.6 Although most studies in the hematopoietic circle have focused on leukocyte maturation, the highest amounts of circRNAs appear in erythrocytes and platelets, motivating recent attention to thrombopoiesis.4
Some circRNAs modulate stability by forming an RNA duplex with a cognate mRNA, whereas other circRNAs cooperate with proteins. In megakaryocytes, circFUT8 falls into the latter category, cooperating with IGF2BP2. This interaction is similar to the previously characterized complex of circNSUN2, IGF2BP2, and HMGA2 mRNA involved in colorectal cancer.7
Wu et al studied whether circFUT8 in megakaryocytes played a role in thrombopoiesis. They found that circFUT8 helps guide actin cytoskeletal rearrangement to produce proplatelets by regulating TNS1 expression. The functional importance of circFUT8 became clear when they knocked down levels of circFUT8 in megakaryocytes, which resulted in decreased TNS1 protein expression, decreased F-actin, and decreased proplatelet production. The functional role of circFUT8 was then validated in vivo, where they observed that the inhibition of circFUT8 in a murine model reduced platelet counts and, as a consequence, prolonged tail bleeding times. Thus, by promoting TNS1 expression to regulate actin polymerization in megakaryocytes, circFUT8 plays a functional role in thrombopoiesis.
With this study, circFUT8 joins the line of circRNAs known to play either positive or negative roles in regulating cytoskeleton dynamics. For instance, the circRNA family member, circASH2, has previously been shown to target the actin binding protein tropomyosin 4 to suppress cytoskeletal reorganization, acting as a negative regulator of migration under physiological conditions.8 In the cardiovascular system, circRNA cZfp292 has been shown to play a requisite role in endothelial cell cytoskeletal reorganization and alignment with shear.9 Moreover, a microarray analysis of M1 and M2 macrophages found that circFUT8 was upregulated in the M1 phenotype, suggesting a possible role in macrophage polarization and inflammation.10 Thus, the significance of circFUT8 in particular appears to encircle a range of cell types and functions, and holds potential as a biomarker and therapeutic target for blood disorders. Coming full circle, the study by Wu et al adds a new role for circFUT8 in the mechanisms of platelet production.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal