In this issue of Blood, Kruer et al1 report that upregulation of the nuclear exporter (XPO1) is central to acquired resistance in TP53-mutant myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) following treatment with the combination of azacytidine (AZA) and the p53 reactivator eprenetapopt. The preclinical findings present a strong case for investigating clinically available XPO1 inhibitors in combination with eprenetapopt for patients who progress and have limited therapeutic options.
Myeloid malignancies with TP53 mutations represent a distinct disease group with dismal outcomes. Treatment of this patient group is extremely challenging because currently there are no approved specific, targeted therapies available to eliminate the TP53-mutated clone; therefore, hematopoietic stem cell transplant (HSCT) remains the only potentially curative treatment.2 Eprenetapopt is a promising, first-in-class, small molecule that restores p53 function in tumors expressing mutant TP53.3,4 In AML cells with TP53 missense mutations the combination of azacytidine (AZA) and eprenetapopt is highly synergistic3 and this combination has induced impressive molecular remissions for TP53-mutant MDS/AML in pivotal clinical trials; however, relapse inevitably occurred unless patients received HSCT.4,5 The present study is, therefore, of high importance, revealing potential therapeutic strategies that may be applied once resistance develops.
As a critical step for the investigation of novel therapies for TP53-mutant MDS the authors generated a cohort of 9 patient-derived xenografts (PDX) with features of TP53-mutant MDS, including 7 from very high-risk patients with TP53 missense mutations. As the impact of TP53 missense mutations in myeloid malignancies can vary considerably,2 this panel provides a key resource. Next, the authors show that in patients treated with AZA plus eprenetapopt, progression is linked to the reemergence of a TP53-mutated clone initially depleted by treatment, suggesting resistance arises from changes in sensitivity of the original clone, rather than new clonal outgrowth. The initial lead for XPO1 as an important player in the development of resistance came from a whole genome CRISPR/Cas9 knockout screen using a cell line model of TP53-mutant AML expressing the dominant-negative mutation, TP53R175H. This identified XPO1, encoding exportin-1, which is responsible for the nuclear export of many molecules central to oncogenesis and associated with resistance to chemotherapy and targeted therapies.6 XPO1 mRNA and protein is upregulated in AZA- and AZA-eprenetapopt–resistant AML cell lines expressing mutant TP53, whereas XPO1 depletion only sensitized cells to eprenetapopt or combination treatment including eprenetapopt.
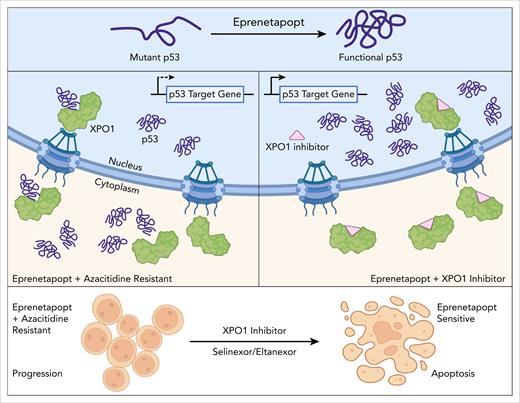
The authors showed that mechanistically mutant p53 is localized in the cytoplasm in resistant TP53-mutant AML cells, but redistributes to the nucleus in the presence of the XPO1 inhibitor, leptomycin B. A strength of this study is the leveraging of primary patient material, results from which are integrated with those from cell lines to develop a working model for the development of AZA-eprenetapopt resistance (see figure). A key finding is that XPO1 mRNA is upregulated in MDS stem and progenitor cell populations after AZA-eprenetapopt combination treatment, with increased XPO1 protein and reduced p53 nuclear/cytoplasmic (N/C) ratio at progression.
The findings presented by Kruer et al suggest a mechanism whereby, in the context of AZA-dependent XPO1 overexpression, the functional activity of p53 and transcription of its key downstream genes that have been restored by eprenetapopt are abolished via XPO1 (exportin-1)-mediated transport out of the nucleus, thus conferring resistance to the combination therapy. Treatment with XPO1 inhibitor is a proposed therapeutic approach for restoring nuclear p53 activity in combination with eprenetapopt. Figure created with BioRender.com. D’Andrea R. (2025) https://BioRender.com/te49jr2.
The findings presented by Kruer et al suggest a mechanism whereby, in the context of AZA-dependent XPO1 overexpression, the functional activity of p53 and transcription of its key downstream genes that have been restored by eprenetapopt are abolished via XPO1 (exportin-1)-mediated transport out of the nucleus, thus conferring resistance to the combination therapy. Treatment with XPO1 inhibitor is a proposed therapeutic approach for restoring nuclear p53 activity in combination with eprenetapopt. Figure created with BioRender.com. D’Andrea R. (2025) https://BioRender.com/te49jr2.
Given availability of the FDA-approved XPO1 inhibitor, selinexor and promising indications for application of these in hematologic malignancies,7 the authors next tested inhibition of XPO1 as an approach to restore nuclear p53 activity in eprenetapopt-resistant, TP53-mutant MDS/AML. The synergy observed in vitro with eprenetapopt and XPO1 inhibitor in AML cell lines expressing TP53 mutants, but not wild-type controls, is intriguing given that eprenetapopt activity in tumor cells is independent of mutant TP538 and suggests a mechanism unique to TP53-mutant MDS/AML. Most encouragingly, testing using the MDS PDX models demonstrated reduced engraftment of patient samples treated with eprenetapopt in combination with the second generation XPO1 inhibitor eltanexor.6 Further studies are needed to directly determine the reduction of the TP53-mutant cells via measurement of TP53 VAF at the start and end of the combination treatment.
These findings raise several questions:
Is there a difference in response to the combination of eprenetapopt and XPO1inhibitor in the complete absence of TP53 wild-type function, compared to the dominant-negative cell line models used here?
What are the relative roles of transcriptional and posttranscriptional regulation of XPO1, given that XPO1 gene methylation is not changed upon treatment with AZA?
What is the mechanism of cell death induced by the combination of eprenetapopt and XPO1 inhibitor? Cell death mechanisms vary depending on eprenetapopt dose and cell type.8 A more complete understanding of this may suggest approaches to enhance efficacy. A DNA damaging agent may provide optimal therapeutic benefit for this combination given the data showing that daunorubicin maximizes the p53 response of eprenetapopt. Furthermore, MDM2 inhibitors have been recently shown to work synergistically with another p53 reactivator.9
These findings point to a new clinical approach that can be rapidly tested for treatment of TP53-mutant MDS/AML. Appropriate correlative studies will be needed to test potential biomarkers such as TP53 mutant VAF, p53 level or N/C ratio, and XPO1 expression, which may prove helpful in selecting patients for treatment. More comprehensive testing of combinations of XPO1 inhibitors with p53 reactivators in clinical development10 will be greatly facilitated by the panel of PDX generated in this study. To achieve durable responses for TP53-mutant MDS/AML, it will be necessary to investigate approaches to overcome several reported mechanisms of XPO1-inhibitor resistance.7 Future studies will also determine the broader clinical relevance of this resistance mechanism for other poor-outcome, TP53-mutant hematologic malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal