In this issue of Blood, Mellema et al1 identify a fascinating mechanism regulating platelet reactivity in the context of inflammatory bowel disease (IBD). Patients with IBD are at a significantly increased risk of experiencing a thrombotic event during the disease compared to healthy individuals.2,3 Therefore, prophylactic anticoagulation is recommended by several clinical guidelines, especially in high-risk scenarios such as active disease or hospitalization.4 The mechanisms by which IBD promotes coagulation—for instance through elevated lipopolysaccharide levels from gut barrier dysfunction—are only partially understood.5
In this context, Mellema et al introduce a novel concept by identifying the C-type lectin homolog receptor layilin as a platelet receptor that binds the glycocalyx component hyaluronan (HA), thereby modulating platelet reactivity in the setting of IBD.6 These findings significantly advance our understanding specifically of the pathophysiology of IBD-associated thrombosis and of platelet physiology in general. Binding to glycocalyx-localized HA raises the threshold for platelet activation, thereby requiring stronger stimuli to initiate clotting.
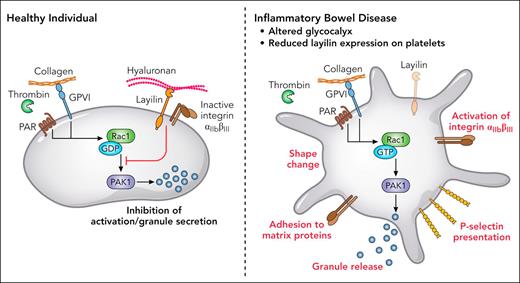
Mechanistically, layilin colocalizes with the fibrinogen receptor integrin αIIbβ3 at the platelet membrane and interacts intracellularly with the cytoskeletal adapter protein talin and merlin.
Through this interaction, layilin modulates the activity of the small Rho GTPase Rac1 and its downstream effector, p21-activated kinase 1, both of which are critical for platelet shape change and granule secretion. In this way, layilin and merlin act as negative regulators of platelet activation by dampening intracellular GTPase signaling cascade triggered by thrombin or collagen (see figure).
In healthy conditions, layilin colocalizes with integrin αIIbβ3, detects HA of the glycocalyx, and mitigates Rac1/p21–activated kinase 1 (PAK-1) signaling in platelets, preventing activation and granule secretion. Under inflammatory conditions in patients with IBD with altered vascular glycocalyx and reduced layilin expression, platelets react to low concentrations of thrombin and collagen with shape change, P-selectin presentation, increased integrin αIIbβ3 affinity to matrix proteins, and granule secretion. Professional illustration by Patrick Lane, ScEYEnce Studios.
In healthy conditions, layilin colocalizes with integrin αIIbβ3, detects HA of the glycocalyx, and mitigates Rac1/p21–activated kinase 1 (PAK-1) signaling in platelets, preventing activation and granule secretion. Under inflammatory conditions in patients with IBD with altered vascular glycocalyx and reduced layilin expression, platelets react to low concentrations of thrombin and collagen with shape change, P-selectin presentation, increased integrin αIIbβ3 affinity to matrix proteins, and granule secretion. Professional illustration by Patrick Lane, ScEYEnce Studios.
Notably, in healthy vessels in the presence of layilin/merlin activating signaling in platelets is permanently restrained. It is thus conceivable that in inflammatory settings where the glycocalyx is damaged and HA levels are reduced, layilin-mediated inhibition is attenuated, allowing platelets to respond more readily to activating cues.
Additionally, the authors demonstrate an additional factor contributing to platelet hyperreactivity in IBD beyond altered glycocalyx sensing. In layilin knockout mice, platelets exhibit heightened responsiveness to thrombin, with elevated P-selectin expression and increased integrin αIIbβ3 affinity. This hyperreactivity results from the loss of layilin’s inhibitory function. Importantly, the authors also show that subjects with IBD express reduced levels of layilin on their platelets compared to healthy individuals. Thus, the disrupted glycocalyx in inflammatory areas and reduced layilin expression likely act in concert to drive thrombotic complications in IBD.
Based on these findings, the authors propose the GTPase Rac1 as a potential target for antithrombotic therapy. Rac1 has also been investigated in cancer therapy, since it is implicated in cancer development, proliferation and metastasis.7 However, Rac1 is ubiquitously expressed and involved in multiple cellular processes. Rac1 regulates cellular motility, the integrity of the blood-brain barrier, and plays a role in the dynamics of synaptic connectivity in neurons.7 Thus, systemic Rac1 inhibition may lead to severe adverse effects, potentially outweighing its benefits in reducing thrombotic risk. Inhibition of Rac1 as an antithrombotic strategy, should therefore be regarded cautiously.
In summary, this study identifies a new mechanism of platelet hyperreactivity through reduced layilin expression, which is thought to play a major role in the context of IBD-related thrombosis. Layilin modulates platelet reactivity by interacting with HA in the glycocalyx and increases the threshold for platelet activation through inhibition of Rac1 signaling. In areas of damaged glycocalyx, platelet activation is facilitated, potentially contributing to thrombotic complications. Beyond IBD, it is plausible that layilin also regulates platelet activity in other inflammatory scenarios. To answer this question, further research is necessary. Similarly, molecules, capable of enhancing layilin activity or stabilizing its interaction with merlin may represent effective antithrombotic drugs with fewer side effects than ubiquitous Rac1 inhibition. This could be relevant not only for IBD but also in a broader thrombotic context.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal