In this issue of Blood, Kumar et al1 show that autoantibodies against the plasma protein β2-glycoprotein I (β2GPI) in antiphospholipid syndrome (APS) recognize 2 distinct epitopes in the first domain of the protein. This work both challenges the prevailing view of a single pathogenic “hot spot” and provides mechanistic insight into how these antibodies engage their target.
APS is an autoimmune disorder defined by the occurrence of thrombosis or pregnancy-associated complications in patients persistently positive for antiphospholipid antibodies (aPL). These can be one of 3 different types: anticardiolipin antibodies, anti-β2GPI antibodies, or antibodies that prolong coagulation tests in a phospholipid-dependent manner; so called lupus anticoagulant.2 Since the recognition of β2GPI as one of the primary antigens in antiphospholipid syndrome in 1990,3 the protein has been the subject of intense structural and functional study. β2GPI consists of 5 highly homologous complement-type domains, with the fifth structurally distinct and responsible for phospholipid binding. Crystallography4 and small angle X-ray scattering5 revealed the protein adopts a flexible elongated conformation, with both the first and the fifth domain equally solvent-exposed. Although antibodies against multiple domains of β2GPI have been described, only antibodies against domain I were shown to associate with thrombotic risk.6 Early mapping studies identified Arg39 in domain I as critical for autoantibody binding,6 leading to the view that pathogenic antibodies converge there.
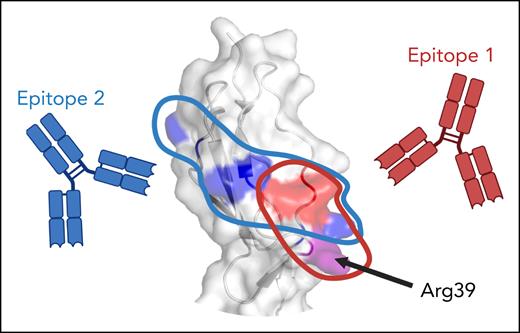
Kumar et al revisited this model using a comprehensive panel of β2GPI variants, consisting of domain deletion mutants as well as variants in which all charged and exposed hydrophobic residues in domain I were individually mutated. With this toolbox, they mapped the binding epitope of MBB2, an archetypal patient-derived monoclonal antidomain I antibody, previously shown to have prothrombotic properties in a rat model.7 MBB2 bound a discontinuous conformational epitope centered on Arg39 (epitope 1; see figure), consistent with prior work. However, when the same approach was applied to samples from triple-positive thrombotic APS patients, a more complex picture emerged. Although all patient antibodies required properly folded domain I, most recognized an alternative epitope (epitope 2) not involving Arg39, whereas some displayed MBB2-like reactivity. Strikingly, patients with MBB2-like reactivity were more likely to have obstetrical problems in addition to thrombosis, raising the possibility that fine epitope specificity influences clinical phenotype. If confirmed in larger cohorts, this diversity in binding epitopes could hold promise for risk stratification based on antibody profile.
Epitope specificity of antidomain I antibodies in triple-positive antiphospholipid syndrome. Antibodies against domain I of β2-glycoprotein I bind 1 of 2 specific epitopes: MBB2-like epitope 1, comprising a discontinuous conformational epitope including Arg39, or epitope 2, stretching across domain I, but not including Arg39. Crystal structure (PDB: 1C1Z) was visualized with PyMOL molecular graphics system. Figure created with BioRender.com. Urbanus R. (2025) https://BioRender.com/3ub2df7.
Epitope specificity of antidomain I antibodies in triple-positive antiphospholipid syndrome. Antibodies against domain I of β2-glycoprotein I bind 1 of 2 specific epitopes: MBB2-like epitope 1, comprising a discontinuous conformational epitope including Arg39, or epitope 2, stretching across domain I, but not including Arg39. Crystal structure (PDB: 1C1Z) was visualized with PyMOL molecular graphics system. Figure created with BioRender.com. Urbanus R. (2025) https://BioRender.com/3ub2df7.
The study also addressed a longstanding paradox in APS: despite high antibody titers, circulating β2GPI-immune complexes are rarely detected and plasma β2GPI levels remain normal. Given the association of antidomain I antibodies with thrombosis, this observation has puzzled investigators for decades. Several hypotheses, including interdomain interactions between the first and fifth domain and conformational changes,8 were put forward. Kumar et al provide an alternative explanation: MBB2 binds β2GPI weakly in solution, making immune complex formation unlikely. However, when β2GPI is immobilized on a surface, MBB2 binding increases dramatically, highlighting the role of avidity in stabilizing antibody-antigen interactions and underscoring the importance of surface context as a driver of pathogenicity in APS. Importantly, although these observations do not exclude a role for conformational change, they suggest that binding strength and concentration are equally important drivers of antibody binding.
Finally, this study reinforces the heterogeneity of anti-β2GPI antibodies. Although MBB2 displays many features of pathogenic aPL, it lacks lupus anticoagulant activity. The relationship between anti-β2GPI antibodies, the lupus anticoagulant phenomenon and thrombosis is well established,9 but appears not to be universal for all anti-β2GPI antibodies. Indeed, other human-derived monoclonal antidomain I antibodies were reported to have modest lupus anticoagulant activity.10 Whether these antibodies bind epitope I, Arg39, or another region entirely remains to be determined.
Conflict-of-interest disclosure: R.T.U. reports research funding from Hemab Therapeutics, paid to the institution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal