In this issue of Blood, Tuazon et al report long-term results from their phase 1 study of FCARH143, a fully human B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR) T-cell therapy that achieved a 100% overall response rate in heavily pretreated patients with relapsed/refractory (R/R) multiple myeloma (MM).1
The study (NCT03338972) investigated FCARH143, a BCMA CAR T cell with a 4-1BB costimulatory domain delivered in a predefined CD4+:CD8+ ratio. The therapy demonstrated remarkable efficacy, with 68% of patients achieving stringent complete response (sCR) at a median follow-up of 67.3 months. The median progression-free survival (PFS) was 15.5 months, with median overall survival (OS) reaching 32 months. These results are particularly noteworthy given the challenging patient population who had received a median of 8 prior lines of therapy. Additionally, 80% of patients were triple-class refractory, and 44% presented with extramedullary disease (EMD).
BCMA-targeted CAR T-cell therapies have transformed treatment options for RR MM.2 BCMA, which belongs to the tumor necrosis factor receptor family, appears on virtually all malignant plasma cells, making it an excellent immunotherapy target. Although the Food and Drug Administration (FDA) has approved idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel), both with excellent efficacy results in both early and late setting of R/R MM, questions remain about their long-term efficacy and long-term safety profiles.3-6
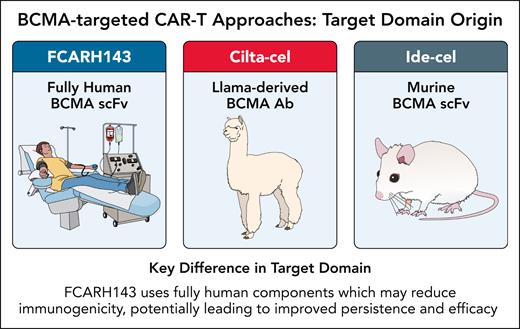
The FCARH143 product consists of engineered T cells that target BCMA using a completely human-derived CAR construct (see figure). This therapy is specifically designed with an equal proportion of helper CD4+ and cytotoxic CD8+ CAR T cells (1:1 ratio) in the final infusion preparation. Additionally, these modified T cells express a shortened, nonfunctional version of human epidermal growth factor receptor (EGFR) on their surface, which serves as a biomarker allowing identification and tracking of the cells that have successfully incorporated the CAR construct. Unlike some CAR T-cell constructs that incorporate murine components, the fully human design potentially reduces immunogenicity, which should translate to improved persistence and efficacy. Additionally, FCARH143 is manufactured to deliver a precise 1:1 ratio of CD4+:CD8+ CAR T cells, a formulation strategy aimed at optimizing both helper and cytotoxic T-cell functions.
BCMA-targeted CAR T-cell approaches: target domain origin. AB, antibody; scFv, single-chain variable fragment. Professional illustration by Patrick Lane, ScEYEnce Studios.
BCMA-targeted CAR T-cell approaches: target domain origin. AB, antibody; scFv, single-chain variable fragment. Professional illustration by Patrick Lane, ScEYEnce Studios.
Regarding the safety of FCARH143, cytokine release syndrome (CRS) occurred in 84% (n = 21), with 64% (n = 16) experiencing grade 1, 16% (n = 4) experiencing grade 2%, and 4% (n = 1) experiencing grade 3. Immune effector cell-associated neurotoxicity syndrome was observed in 5 patients or 20% (2 patients grade 1, 3 patients grade 2) and no cases of grade 3. No delayed neurotoxicities, including movement disorders and cranial nerve palsies, were reported. One patient developed Parkinson disease 2 years post-FCARH143, which was determined to be unrelated to treatment after a comprehensive neurologic evaluation.
Long-term data from other BCMA-targeted CAR T-cell therapies in R/R MM have been reported, including the LCAR-B38M (LEGEND-2) trial, which was a first-in-human phase 1 study of LCAR-B38M conducted in China and subsequently developed as Cilta-cel in the United States.7 This phase 1 study involved 74 patients with a median follow-up of 65.4 months. In terms of safety, LCAR-B38M was associated with a high rate of grade ≥ 3 CRS (10%). In the LCAR-B38M cohort, 5.4% developed secondary solid cancers between 8 and 32 months postinfusion, with no cases of secondary hematological malignancies. One patient treated with FCARH143 developed myelodysplastic syndrome 57 months postinfusion. Monitoring for secondary malignancies will be extremely important given early signals of increased incidence of secondary hematological malignancies in CARTITUDE-4 study. As certain safety signals were not initially seen in clinical studies but were found later in real-world use, it is important to understand that large numbers of patients mut be treated to determine the actual safety profile of products in early development.8
An intriguing aspect of this trial was the minimal dose dependency of responses, with patients receiving as few as 50 × 106 CAR T cells achieving comparable outcomes to those receiving higher doses. This contrasts with ide-cel, where doses below 150 × 106 have been associated with suboptimal results, suggesting potentially enhanced potency of the fully human construct.
The study also highlights the critical impact of EMD on outcomes. Patients with true EMD had significantly shorter PFS and OS (8.1 vs 23.8 months and 17.7 vs 58.4 months, respectively) compared with those without, underscoring the continued challenge of treating this high-risk feature. EMD is often defined differently in clinical trials, which is a significant issue, as outcomes of paraskeletal EMD differ from organ-related EMD.
Although the trial demonstrates promising results, several questions remain. Given the small cohort size (n = 25) and single-center design, confirmation in larger, multicenter trials will be essential. The role of this therapy in the context of approved BCMA-directed CAR T cells and bispecific antibodies products requires further elucidation.9 The absence of a predetermined maximum tolerated dose or recommended phase 2 dose leaves some ambiguity about optimal dosing, though the authors suggest 450 × 106 cells as reasonable based on safety data.
Looking ahead, this study emphasizes several important directions for BCMA-targeted CAR T-cell therapy. Several specific aspects of FCARH143 warrant further investigation in larger trials. The truncated EGFR safety switch, although not used in this study, provides a theoretical advantage for managing severe toxicity through administration of cetuximab or similar anti-EGFR antibodies. Additionally, the outpatient administration for most patients should reduce health care utilization and improved patient experience. The authors’ plans to explore dual targeting with GPRC5D (BMS-986453) may address antigen loss as a resistance mechanism and represents an exciting avenue for future research.
In summary, FCARH143 demonstrates the potential advantages of a fully human BCMA targeted CAR T-cell therapy. The extended follow-up period allows meaningful assessment of durability, with some patients maintaining responses beyond 3 years. As the field continues to refine CAR T-cell approaches for MM, FCARH143 offers a promising template for balancing efficacy, safety, and manufacturing consistency. Whether this approach will ultimately prove superior to current FDA-approved options remains to be determined. Another study that investigated fully human BCMA CAR T-cells reported promising results,10 and the findings presented by Tuazon and colleagues provide compelling rationale for continued development of fully human BCMA-targeted CAR T-cell therapies.
Conflict-of-interest disclosure: S.A.H. reports receiving consulting fees from Janssen and Sanofi and research funding from Pfizer and Janssen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal