In this issue of Blood, Kotrová et al1 profile the landscape of immunoglobulin (IG) and T-cell receptor (TR) rearrangements in more than 1000 samples at diagnosis from patients with acute lymphoblastic leukemia (ALL), revealing a heightened stage of immunogenetic development in pediatric ALL compared with adult patients.
During normal B- and T-cell development, IG and TR genes undergo a highly regulated process of rearrangements to produce a diverse repertoire of antigen receptors, which are essential for adaptive immune responses. This process is called V(D)J recombination and involves the random “cut and paste” of gene segments—V (variable), D (diversity), and J (joining)—by recombination-activating gene proteins and other enzymes. These rearrangements follow a hierarchical order with the joining of D-to-J segments occurring first, followed by the assembly of a V segment to the pre-rearranged DJ unit.
In B cells, V(D)J recombination occurs first at the IG heavy chain (IGH) gene loci and then at the IG light chain genes, either κ (IGK) or λ (IGL), to produce a functional B-cell receptor. This transition correlates with the developmental progression from pro–B- to pre–B-cell stage, where a complete B-cell receptor is expressed on the cell surface. In T cells, V(D)J recombination occurs at the TR loci: recombination of TR-δ, TR-γ, and TR-β occur at the pro-T stage. TR-α rearrangement occurs later at the double-positive stage. Recombination of TR-δ and TR-γ promotes assembly of a TR-γδ, whereas assembly of TR-β with TR-α forms a TR-αβ.2
Because leukemic transformation can arrest this process at distinct stages of differentiation, the status of V(D)J rearrangement leaves a molecular footprint reflective of the stage at which B/T-cell development is arrested. In B-ALL, early transformations occurring at the pro-B stage may result in incomplete IGH rearrangements (eg, D-J recombination without V). Conversely, later transformations at the pre-B stage or beyond may result in complete IGH rearrangements (V-D-J) alongside IGK/IGL rearrangements. In T-ALL a recent genomic study of over 1300 pediatric samples revealed that T-ALL molecular subtypes are highly tied to the TR maturation stage, spanning a spectrum from immature (pre-TR) to committed (αβ or γδ) stages.3 In this study, single-cell transcriptome and epigenetic normal reference maps were used to determine developmental states in T-ALL transcriptome data. Absence of TR rearrangements suggested a prethymic or early thymic progenitor stage, such as in the case of early T-cell precursor (ETP) ALL and ETP-like ALL subtypes. Early pro/pre-T subtypes were often linked to KMT2A, TLX3, and MLLT10 rearrangements. Subtypes including HOXA9, TLX1, and NKX2-1 often showed TR-β rearrangements without TR-α. Mature T-ALL, conversely, displayed TR-α rearrangements alongside TR-β (eg, subset of TAL1 αβ-like) and TR-γδ, indicating a later arrest (eg, LMO2 γδ-like).
The unique pattern of rearrangements is clinically significant, and it is used to track disease progression by measurable residual disease (MRD) monitoring, assess clonality, and determine the immunogenetic maturation state of leukemic cells. However, a deep analysis of the prevalence of leukemia-associated IG/TR gene rearrangements across different subtypes and age groups is missing.
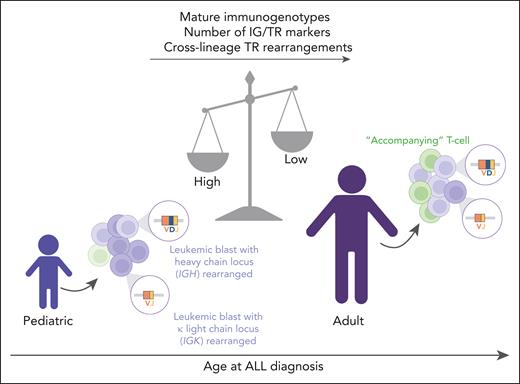
In their study, Kotrová et al examined the landscape of IG/TR rearrangements at diagnosis in a large cohort of pediatric and adult ALL patients (N = 1212) from different molecular subtypes by using the EuroClonality–next-generation sequencing (NGS) amplicon assay,4 which enables high-throughput sequencing of IG and TR rearrangements. This comprehensive analysis revealed 4 main findings (see figure).
Immune gene rearrangement profiles in pediatric and adult ALL. Pediatric patients with ALL show a higher frequency of mature immunogenotypes within both IG and TR genes, a higher number of IG/TR markers, and a higher percentage of cross-lineage TR rearrangements compared with adult patients with ALL. In contrast, older patients with ALL are characterized by a higher frequency of expanded “accompanying” T-cell clones. Figure created with BioRender.com.
Immune gene rearrangement profiles in pediatric and adult ALL. Pediatric patients with ALL show a higher frequency of mature immunogenotypes within both IG and TR genes, a higher number of IG/TR markers, and a higher percentage of cross-lineage TR rearrangements compared with adult patients with ALL. In contrast, older patients with ALL are characterized by a higher frequency of expanded “accompanying” T-cell clones. Figure created with BioRender.com.
First, the maturation stage of the IG/TR rearrangement is age-dependent, with younger ALL patients exhibiting rearrangements at more mature stages of B/T-cell development. This trend was independent of molecular subtype and persisted within patients with the same immunophenotype. Moreover, children showed a higher average number of IG/TR clonal rearrangements compared with adult patients with ALL. The authors speculated that these observations may reflect a distinct origin of ALL in children (eg, from abnormal immune response to infections)5 and adults (eg, from gradual accumulation of mutations).6 However, epidemiologic data were not available to corroborate these correlations.
Second, illegitimate cross-lineage TR rearrangements decrease with age and are less common in KMT2A-rearranged and TCF3::PBX1 leukemias compared with other subtypes. These rearrangements occur when TR loci undergo aberrant V(D)J recombination outside the T-cell developmental context. Cell-dependent epigenetic regulation may determine a lower accessibility of TR loci and explain differences among cell differentiation stages.
Third, IG/TR detection by NGS enables identification of multiple rearrangements and assessment of clonal evolution. Consistent with previous findings, Kotrová et al confirmed that V-DJ recombination drives pro–B-cell evolution, whereas V-replacement is more common in pre-B cells. Interestingly, in both pediatric and adult ALL, clonal evolution of IG gene rearrangements was more common than TR rearrangements. Clonal evolution presents a significant challenge in MRD monitoring as it can result in the modification or complete loss of IG/TR markers over time, increasing the risk of false-negative MRD assessments. Notably, KMT2A-rearranged leukemia cases are particularly prone to IGH clonal evolution.7 Although NGS approaches are highly effective in assessing overall rearrangement prevalence, they average signals across heterogeneous cell populations, masking rare subpopulations. In contrast, single-cell sequencing–based approaches may not only detect multiple V(D)J patterns but assign them to distinct cellular subsets, thus improving clonal evolution interpretation.8
Finally, the authors have revealed a higher frequency of expanded “accompanying” T-cell clones in older patients compared with younger patients. The role of these cells remains unexplored, but recent findings indicate that clonal complete TR-δ rearrangements in B-ALL derive from bystander γδ T-cell clones and their abundance is associated with better MRD response.9 Notably, infiltrating or residual T cells may influence the tumor microenvironment and immune responses.10
In conclusion, Kotrová et al have provided a comprehensive analysis of the prevalence of V(D)J rearrangements across distinct subtypes and age groups of ALL, highlighting the higher immunogenicity maturity in children compared with adults. A crucial next step will be to investigate the clinical impact of specific rearrangement profiles across large, well-defined ALL cohorts, identifying prognostic biomarkers and predictors of therapeutic resistance.
Conflict-of-interest disclosure: I.I. has received a consultation fee from Arima Genomics and travel costs paid by Mission Bio and Takara.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal