In this issue of Blood, Munsch et al1 identify C-type lectin domain family 4 member M (CLEC4M) as a potential new clearance receptor for plasma factor V (FV) levels. They further show that CLEC4M regulates FV clearance by binding and internalizing FV.
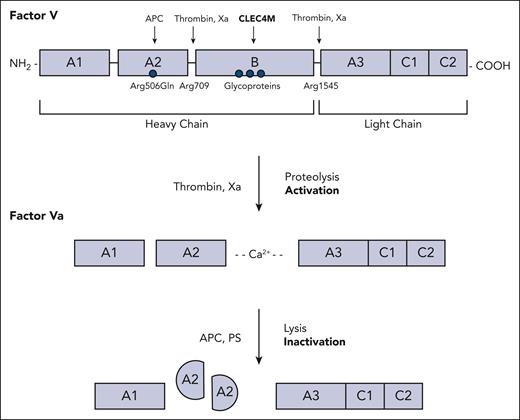
FV is a key regulator of hemostatic balance. It has dual roles, involved in bleeding and thrombosis. Located on 1q24-25, FV is encoded for by the F5 gene and has a domain structure A1-A2-B-A3-C1-C2, which shares 40% homology to FVIII. Approximately 80% of FV circulates in plasma and 20% in platelets.2 Following activation by thrombin and activated FX, FVa converts prothrombin (FII) to thrombin (FIIa) (see figure), which, in turn, catalyzes fibrin deposition and platelet cross-linking to enable platelet plug formation and primary hemostasis.
Schematic diagram of FV and FVa. FV protein structure and regulatory proteins involved in FV activation and FVa inactivation are shown. APC, activated protein C; PS, protein S.
Schematic diagram of FV and FVa. FV protein structure and regulatory proteins involved in FV activation and FVa inactivation are shown. APC, activated protein C; PS, protein S.
Clinically, FV deficiency (Owren disease or parahemophilia)3 is a rare autosomal recessive bleeding disorder occurring in 1 in 1 million people. Caused by >200 loss-of-function mutations in the F5 gene, including missense and nonsense variants, FV deficiency is characterized by mucosal and traumatic bleeding, primarily epistaxis, excessive postpartum and/or menstrual bleeding and, rarely, intracranial bleeding.4 Treatment is fresh frozen plasma, which also replenishes platelet FV. Paradoxically, other F5 mutations may result in thrombosis, the most common of which is Arg506Gln (FV Leiden). Present in 5% of the population, this mutation is associated with resistance to activated protein C, which cleaves and inactivates FVa, and results in a fivefold to sevenfold increased risk of venous thrombosis.5,6
Thus, although FV expresses both procoagulant and anticoagulant functions, FV levels do not predict bleeding or clotting risk. For example, severe FV deficiency is typically associated with bleeding, whereas both high and low FV levels may be associated with thrombosis. In this article, the authors sought to determine regulators of FV plasma levels. As CLEC4M is a clearance receptor for von Willebrand factor (VWF) and FVIII,7,8 regulates FVIII levels in hemophilia A,9 and regulates FVIII and VWF levels in hemorrhage and thrombosis, they hypothesized that CLEC4M might affect FV clearance and FV levels. This hypothesis was further bolstered by the 40% structural homology between FV and FVIII.
The authors conducted a comprehensive bioinformatics analysis, including large-scale proteogenomic and transcriptomic data from original and public data sets, which identified the CLEC4M locus as a clearance receptor for FV. They also identified that the mechanism by which CLEC4M regulates FV clearance and FV levels is through binding FV and promoting its internalization and lysosomal degradation, similar to the mechanism already identified for the regulation of VWF and FVIII through CLEC4M clearance.10
They also identified 26 new proteins and loci involved in the regulation of plasma FV levels by using a large genome-wide association study (GWAS) meta-analysis on plasma FV activity from individuals from 5 cohorts. The resulting meta-analysis had a sample 8 times larger than previous GWASs, with 4373 individuals from the LURIC, MARTHA, MEGA, and RETROVE cohorts in the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium and MARTHA12 study.1
So, what is the clinical significance of the CLEC4M role in FV clearance role and regulation of FV levels?
First, knowledge of this new mechanism of CLEC4M clearance of FV helps provide a better understanding of genetic modifiers of FV pharmacokinetics (PK) and mechanistic insight into pathways that regulate FV clearance. This could be helpful in developing personalized treatment of FV-deficient patients, in predicting PK of new therapies for FV-related disorders, or potential therapeutic targets to increase the in vivo availability of FV.
Second, these data also support the need to consider the potential effects of F5 variants when developing new therapies for those with FV-related disorders. The effects of CLEC4M on FV PK in FV-related disorders should also be considered (eg, following fresh frozen plasma infusion), similar to the recognized impact of CLEC4M on the half-life of infused FVIII concentrates in patients with hemophilia A.9
Third, as the level of FV may indicate either an increased risk of bleeding or thrombosis, genetic variants of CLEC4M that influence FV clearance may potentially modify bleeding or thrombotic risk in patients with FV variants, as observed with the impact of CLEC4M variants on VWF and VIII clearance in risk for bleeding or thrombosis.10
Fourth, these findings open up new avenues to better understand the precision of the physiological processes involved in thrombotic and bleeding disorders, a precision no less compelling than the improvisational 5/4 rhythm of Dave Brubeck’s “Take Five.”
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal