In this issue of Blood, Tranter et al1 identified the mechanosensitive volume-regulated anion channel (VRAC) with its protein subunit LRRC8 (leucine-rich repeat–containing 8) as a promising new target for antithrombotic treatment, elegantly linking early processes of platelet activation, such as morphological shape change, to local thrombosis and clot formation.
Thrombo-occlusive cardiovascular diseases, such as myocardial infarction with subsequent ischemia/reperfusion (I/R) injury and ischemic stroke, remain the major cause of morbidity and mortality worldwide.2 The existing dual antiplatelet therapy that consists of aspirin treatment and a P2Y12-receptor antagonist, such as clopidogrel, prasugrel, or ticagrelor, offers effective antithrombotic treatment regimens and have improved both the prognosis and clinical outcome of patients with cardiovascular diseases. However, despite extensive research efforts and improvements being made,2,3 existing antiplatelet therapies remain limited by potentially life-threatening risks, including excessive hemorrhage. Consequently, there is a substantial and unmet clinical demand for new druggable targets that are capable of efficiently preventing pathologic platelet activation and thrombosis without an increased risk for hemorrhage.2 In particular, signaling pathways that translate early and local restricted platelet activation at sites of flow-limiting atherosclerotic plaques or ruptured plaque lesions with subsequent coronary artery occlusion represent promising targets for new and improved antithrombotic treatments. Currently, mechanosensitive mechanisms in platelets are gaining scientific notice because of their pivotal role in hemodynamics and the role of shear stress in platelet activation and subsequent clot formation. Previous research predominantly examined platelet ion channels with mechanoresponsive and cell volume–regulating properties, such as the transient potential cation channel subfamily V member 4 (TRPV4) and the transmembrane protein 16F (TMEM16F, Ano6), because they regulate thrombosis by translating the mechanical impact into intracellular pathways. Descriptions of mechanosensitive membrane channels remain scarce. Recently, however, the mechanosensitive Piezo 1 channel was characterized in platelet activation and thrombosis4 and the innovative translational approach of genome- and phenome-wide association studies by Tranter et al identified another mechanoresponsive molecule that mediates platelet function. The LRRC8 protein family provides the subunits of a functional VRAC expressed in a wide variety of different cell types, including murine and human platelets, which together with TRPV4 and Ano6 channels, senses changes in cell volume and is crucial for osmoregulation by, for example, facilitating the efflux of chloride ions across the cell membrane.5
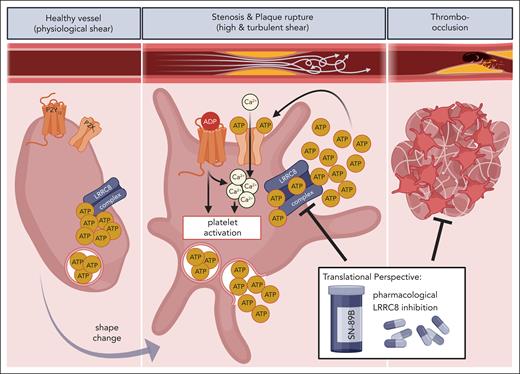
Notably, LRRC8 converts the platelet’s volume and shape change, which are the earliest changes after platelet activation, into auto- and paracrine activation mechanisms. LRRC8 contributes significantly to the local pool of extracellular second-wave mediators, adenosine di-/triphosphate (ADP/ATP), because of its noncanonical function in ATP efflux from platelets upon structural changes (see figure). This is of great clinical relevance. First, these mediators can induce Ca2+ influx via the ATP-gated calcium-permanent ion channel P2X1 or via purinergic signaling that relies on the ADP receptors P2Y1 or P2Y12, thus fostering platelet aggregation and clot formation.6 As rising intracellular Ca2+ levels are the most pivotal prerequisite for platelet activation,2 LRRC8 activation upon platelet stimulation by mechanical forces or diverse agonists affects thrombus formation and vessel occlusion in arterial and venous vasculature. In addition to its relevance in Ca2+-dependent platelet activation and hemostasis, ADP-dependent second-wave signaling is essential for thrombus development and stability under various shear rates.7 In contrast with the existing P2Y receptor antagonists, inhibiting LRRC8 did not increase bleeding or hemorrhage. In this regard, platelet activation by a mechanosensitive release of platelet-derived ATP is limited to sites with pathologic shear forces, including stenoses and atherosclerotic plaque ruptures, both of which contribute significantly to pathologic thrombus formation. However, extracellular ATP is also a well-known mediator of acute inflammatory response, thus promoting vascular- and thromboinflammation via P2Y and P2X receptors8 and feasibly affecting tissue damage in cardiac I/R and myocardial remodeling after acute myocardial infarction.
Considering that platelets are among the first cells that accumulate within the infarcted myocardium, platelet-derived ATP plays a pivotal role in the (post)ischemic myocardial environment by inducing inflammatory responses and mediating cardiac fibrosis via P2Y12.9 Thus, although the protective effects of VRAC impairment in I/R injury were hitherto only associated with attenuated excitotoxicity in neuronal cells and cardiomyocytes,10 the newly discovered role of LRRC8 in platelet activation and thrombosis signifies an increased relevance of VRAC in the field of effective antiplatelet therapy in thrombo-inflammatory diseases, which is demonstrated by the substantial reduction of thrombus formation without deregulatory effects on homeostasis in mice with LRRC8 inhibition or depletion.1
The recent discoveries conclusively identified LRRC8 as a potential highly effective new target for inflammatory disorders of the vasculature and myocardium beyond its encouraging prospects in future antithrombotic treatments (see figure). Placing VRAC inhibition into the context of improved treatment regimens of inflammatory and thrombo-occlusive cardiovascular diseases is a most intriguing and novel perspective cultivated by Tranter et al by optimizing an existing VRAC inhibiting compound. This optimized compound, designated SN-89B, exhibits a predicted decrease in plasma membrane protein binding but, more significantly, also demonstrates an efficient antiplatelet function without adverse bleeding in vivo. Ultimately, it will be most interesting to observe the first clinical trials of SN89B as a new potential antiplatelet therapy considering its promising effects on local platelet activation and clot formation and to investigate new avenues for clinical applications.
Schematic overview of LRRC8 activation and ATP-release with subsequent platelet activation, thrombo-occlusion, and clinical manifestation upon mechanical shear stress. Although in resting platelets the LRRC8 complex (VRAC) is inactive, mechanical shear stress at the sites of atherosclerotic stenosis or plaque rupture mediates LRRC8-dependent release of ATP with subsequent autocrine and paracrine platelet activation via purinergic (P2X1, P2Y12) signaling. Shear-induced platelet activation leads to clot formation and cardiovascular disorders, such as thrombosis, ischemic stroke, and myocardial thromboinflammation, which is potentially averted by pharmacologic LRRC8 inhibition. The figure was created with BioRender.com. Münzer P. (2025) https://biorender.com/72ra0hf.
Schematic overview of LRRC8 activation and ATP-release with subsequent platelet activation, thrombo-occlusion, and clinical manifestation upon mechanical shear stress. Although in resting platelets the LRRC8 complex (VRAC) is inactive, mechanical shear stress at the sites of atherosclerotic stenosis or plaque rupture mediates LRRC8-dependent release of ATP with subsequent autocrine and paracrine platelet activation via purinergic (P2X1, P2Y12) signaling. Shear-induced platelet activation leads to clot formation and cardiovascular disorders, such as thrombosis, ischemic stroke, and myocardial thromboinflammation, which is potentially averted by pharmacologic LRRC8 inhibition. The figure was created with BioRender.com. Münzer P. (2025) https://biorender.com/72ra0hf.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal